Figures & data
Table 1. Characteristics of the study participants.
Figure 1. The frequency of reported adverse events within 7 days of the first, second, and third doses of a COVID-19 vaccine among the study participants. Notably, 99% of the participants received the ChAdOx1 vaccine as their first and second doses, and the mRNA-1273 vaccine accounted for 97% of their third doses. The adverse events are presented in descending order of the first COVID-19 vaccine.
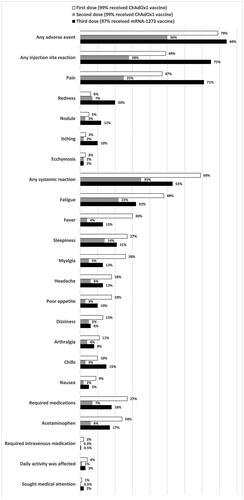
Table 2. Comparison of the frequency of adverse events following COVID-19 vaccination among the three doses.
Table 3. Comparison of adverse events which resolved ≤2 days among the study participants who had the adverse events following COVID-19 vaccination.
Table 4. Variables associated with the adverse events following three doses of the COVID-19 vaccination.
Data availability statement
Individual-level deidentified participant data will be made available upon request by emailing the corresponding author. The data will be available for 3 years after publication.
