Figures & data
Figure 1. Flow chart of hAMSC treatment and skin regeneration, quality of life in the CUA patient. (A) Flow chart of hAMSC treatment. (B-E) Healing process of the skin lesions on the right thigh during the course of hAMSC treatment. (B) The anterior of right thigh presenting with large areas of irregular black scab, superficial ulceration and slight exudation before hAMSC treatment. (C) Patchy shallow ulcer covered with granulation tissues, pale yellow necrotic tissues and crusting in the center after hAMSC treatment for 2 months and debridement. (D) Striated scar with reddish dry ulcer in the center, pigment reduction in the peripheral, dilated blood vessels and a small amount of local crusting after hAMSC treatment for 9 months. (E) Fully regenerated skin accompanied with irregularly shaped scar, localized hypopigmentation and few scabs in the right thigh after hAMSC treatment for 20 months. (F) The left knee presenting with large black eschar and scattered deep ulcers before treatment. (G) Scar healing on the left knee with hypopigmentation after hAMSCs for 15 months. (H-I) Wound assessment based on BWAT(H) and quality of life evaluated by Wound-QoL(I) after hAMSC treatment for 15 months. hAMSC: human amnion-derived mesenchymal stem cell; CUA: calcific uremic arteriolopathy; BWAT: Bates-Jensen Wound Assessment Tool; Wound-QoL: Wound-Quality of Life.
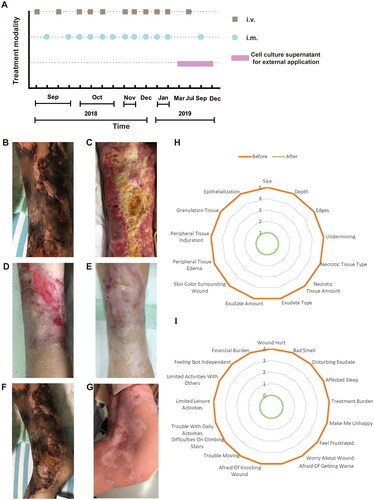
Figure 2. The levels of blood coagulation-related markers of CUA patient before and during the course of hAMSC treatment. (A) Platelets, (B) Fibrinogen, (C) D-dimer and (D) CRP were followed up. The rectangular shaded area represented the normal reference range for each index and the recommended ranges were based on the normal reference values of laboratory tests. CUA: calcific uremic arteriolopathy; hAMSC: human amnion-derived mesenchymal stem cell; CRP: C reactive protein, m: month, d: day, w: week, y: year.
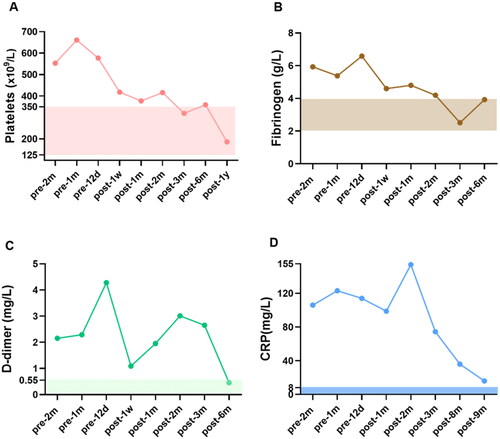
Figure 3. H&E staining of skin biopsy from the calciphylaxis patient before and during hAMSC treatment. (A) Specimen of biopsy obtained from the margin of an ulcer on the thigh, presented exfoliation of epidermis, necrosis, and extensive inflammatory cells infiltration around microvessels (black arrow) before hAMSC treatment (200×). (B) After hAMSC treatment for 1month, skin biopsy showed regenerative tissue with reduced inflammation and absence of epidermis (200×). (C) Intact epidermis, well-aligned collagen without visible inflammatory reaction after hAMSC treatment for 20 months (400×). H&E: Hematoxylin and eosin; hAMSC: human amnion-derived mesenchymal stem cell.
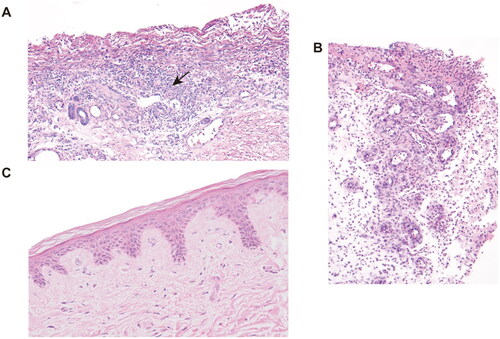
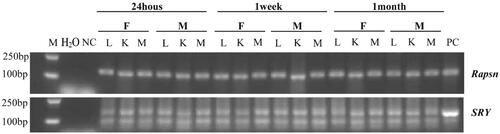
Figure 4. Tissue distribution of hAMSC DNA in C57BL/6 mice after 24 h, 1 week and 1 month of hAMSC infusion. M: DNA gradient labeling; H2O: sterile water; NC: negative control (AMSC10 cells cultured in vitro in the Rapsn primer amplification system and mice muscle tissues without injection of AMSC10 cells in the SRY primer amplification system); PC: positive control (mice muscle tissues without injection of AMSC10 cells in the Rapsn primer amplification system and AMSC10 cells cultured in vitro in the SRY primer amplification system). F: female mice; M: male mice; L: lung; K: kidney; M: muscle, Rapsn: receptor-associated protein of the synapse; SRY: sex determining region Y; hAMSC: human amnion-derived mesenchymal stem cell.