Figures & data
Table 1. Characteristics of clinical studies included in this systemic review.
Table 2. Characteristics of animal studies included in this systemic review.
Figure 2. Changes in the abundance of the gut microbiota in the LN in the clinical and animal studies. The abundance of the phylum Proteobacteria, genus Streptococcus, and species Ruminococcus gnavus were enriched in LN. The F/B ratio was decreased in the LN. F/B, Firmicutes/Bacteroidetes. LN, lupus nephritis.
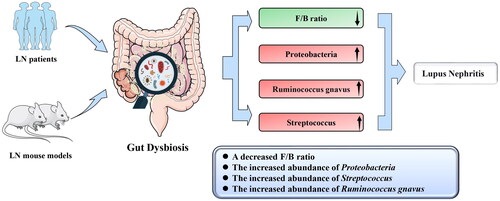
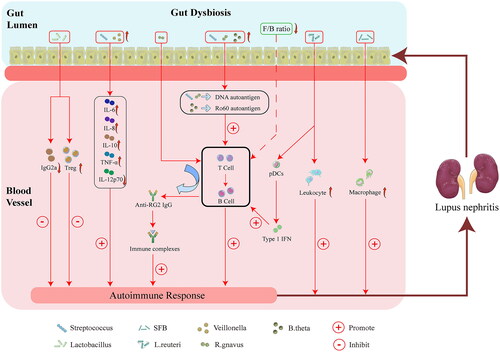
Figure 3. Possible mechanism linking gut microbiota dysbiosis to LN. Alteration of specific microbial taxa may contribute to the pathogenesis and progression of LN through the following four factors. First, the alteration of specific microbial taxa can induce LN by promoting kidney M2-like macrophage infiltration and leukocyte recruitment. Second, the gut microbiota may contribute to LN by enhancing the autoimmune response. Third, Streptococcus combined with Veillonella can enhance the autoimmune response, including by increasing IL-6, IL-8, IL-10, and TNF-α levels, whereas decreased IL-12p70 may induce LN. Fourth, the alteration of specific microbial taxa can increase the abundance of Tregs, while the decrease in the deposition of IgG2a may alleviate LN. SFB, Segmented Filamentous Bacteria. L. reuteri, Lactobacillus reuteri. R. gnavus, Ruminococcus gnavus. B. theta, Bacteroides thetaiotaomicron. F/B, Firmicutes/Bacteroidetes. pDCs, plasmacytoid dendritic cells. IL-12p70, interleukin-12p70. IL-10, interleukin-10. IL-8, interleukin-8. IL-6, interleukin-6. TNF-α, tumor necrosis factor α. Tregs, regulatory T cells. LN, lupus nephritis.
Supplemental Material
Download PDF (332.1 KB)Data availability statement
All data generated or analyzed in the course of this study are included in the document and its supplementary materials. Further inquiry can be available from the corresponding author.