Figures & data
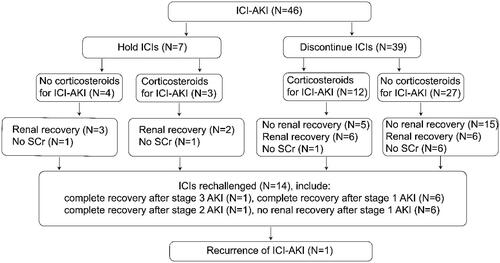
Figure 1. Flow chart of patient selection. a Patients with previous chemotherapy were defined as those with an interval of more than 2 months between subsequent ICIs, including cisplatin/carboplatin, oxaliplatin, gemcitabine, and tegafur. AKI: acute kidney injury; SCr: serum creatinine; ICIs: immune checkpoint inhibitors; ICI-AKI: immune checkpoint inhibitor-associated acute kidney injury.
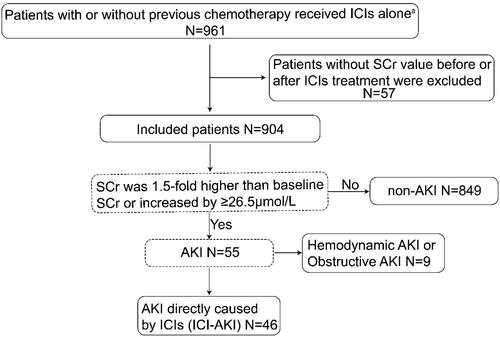
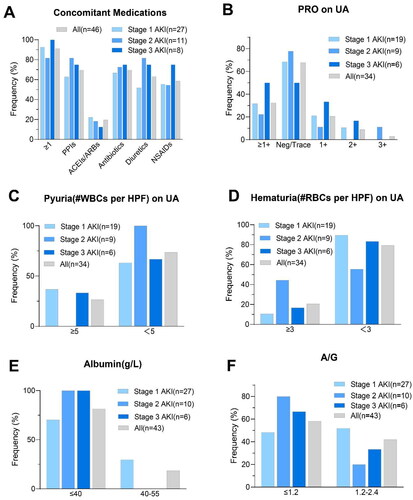
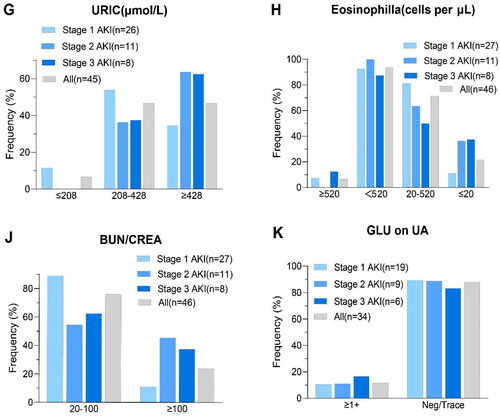
Table 1. Characteristics of the population at baseline. All the data are complete.
Figure 2. Risk factors for ICI-AKI. A total of 895 patients were included; 46 had ICI-AKI and 849 had non-AKI. a Only variables with p < 0.05 according to univariate logistic regression analysis were analyzed via multivariable logistic regression. b Prior chemotherapy was defined as an interval of more than 2 months between subsequent ICIs, including cisplatin/carboplatin, oxaliplatin, gemcitabine, and tegafur. Bold values are statistically significant. ICI-AKI, immune checkpoint inhibitor-associated acute kidney injury; eGFR: estimated glomerular filtration rate; REF: reference; CHD: coronary atherosclerotic heart disease; PPIs: proton pump inhibitors; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin-receptor blockers; NSAIDs: nonsteroidal anti-inflammatory drugs; ICIs: immune checkpoint inhibitors; PD-1: programmed cell death 1; PD-L1: programmed death-ligand 1, OR: odds ratio; CI: confidence interval.
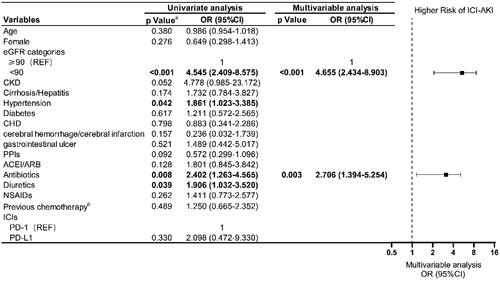
Figure 3. Clinical features of ICI-AKI. (A) The number of weeks between ICIs initiation and ICI-AKI diagnosis. (B) The distribution of ICI-AKI severity according to the Kidney Disease Improving Global Outcomes criteria. (C) The trend in SCr levels (mean ± SEM). The baseline SCr level was defined as the value closest to but before ICIs initiation; the diagnosis SCr refers to the value at which the patient first fulfilled the criteria for ICI-AKI; the peak SCr refers to the highest value during the AKI episode; and ending SCr is the value at the time of renal recovery (for patients with renal recovery) or the most recent value available after AKI diagnosis (for patients without renal recovery). ICIs, immune checkpoint inhibitors; ICI-AKI, immune checkpoint inhibitor-associated acute kidney injury; SCr, serum creatinine.
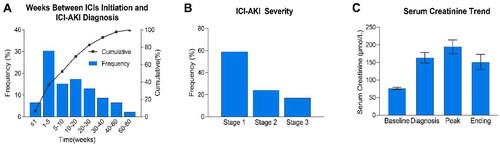
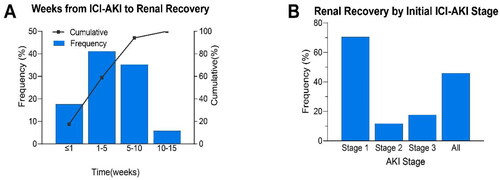
Figure 5. Characteristics of renal recovery in patients with ICI-AKI. (A) Time (in weeks) from ICI-AKI diagnosis to renal recovery. (B) Renal recovery overall and according to initial ICI-AKI stage. Patients without an ending scan of SCr (n = 9) were excluded. ICI-AKI, immune checkpoint inhibitor-associated acute kidney injury; AKI, acute kidney injury.