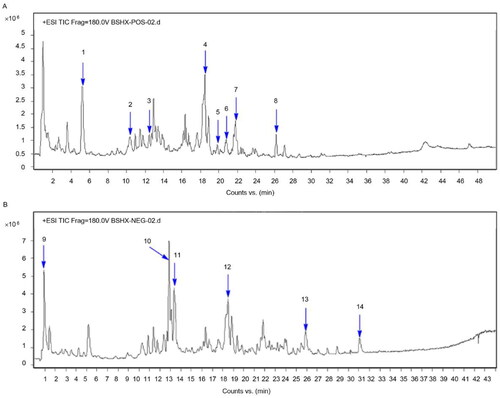
Figures & data
Figure 2. BSHX improves renal function and fibrosis in 5/6 Nx rats. (A) HE (Scale bar, 200 µm. Magnification ×100) and Masson staining (Scale bar, 100 µm. Magnification ×200). The (B) systolic blood pressure, levels of (C) BUN, (D) creatinine, (E) urinary protein, (F) Hyp, (G) TGF-β, (H) MMP2 and MMP9 and (I, J) expression of TGF-β, p-Smad2/3 and Smad2/3. Data represent the mean ± SD (n = 3 or 6). One-way ANOVA followed by Dunnett’s test for multiple comparisons was used. ***p < 0.001 vs. Sham group. !p < 0.05, !!p < 0.01, !!!p < 0.001 vs. the 5/6 Nx group. HE, hematoxylin and eosin; BUN, blood urea nitrogen; Hpy, hydroxyproline; TGF-β, transforming growth factor-beta; MMP2, matrix metalloproteinase 2; MMP9: matrix metalloproteinase 9; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
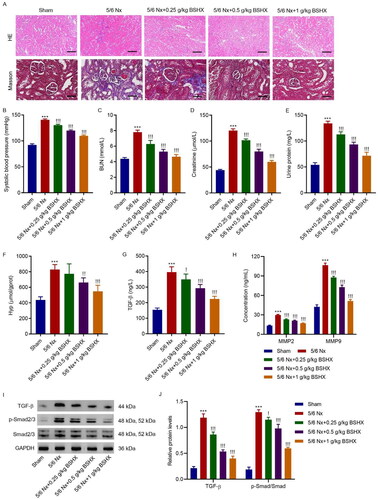
Figure 3. BSHX attenuates oxidative stress and NLRP3 inflammasome activation in 5/6 Nx rats. The activities of (A) MDA, (B) GSH and (C) SOD, (D, E) expression of NLRP3, caspase-1 p20, GSDMD-N, and (F) release of IL-1β and IL-18. Data represent the mean ± SD (n = 3 or 6). One-way ANOVA followed by Dunnett’s test for multiple comparisons was used. ***p < 0.001 vs. Sham group. !p < 0.05, !!p < 0.01, !!!p < 0.001 vs. the 5/6 Nx group. MDA, malondialdehyde; GSH, glutathione; SOD, superoxide dismutase; NLRP3, NOD-like receptor protein 3; GSDMD-N, gasdermin D-N-terminal domain; IL-1β, interleukin-1beta; IL-18, interleukin-18; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
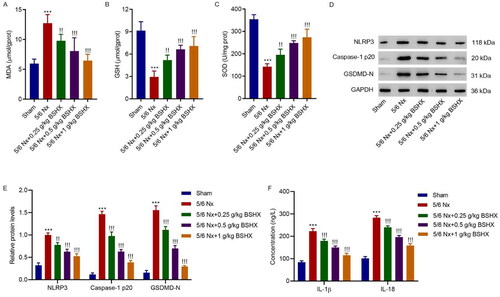
Figure 4. BSHX alleviates Ang II-induced fibrosis and oxidative stress in HK-2 cells. (A) Cell viability in HK-2 cells treated with Ang II (1 µM) in the absence or presence of different concentrations of BSHX (0.1, 0.2, and 0.5 mg/mL). The (B) Hyp, (C) TGF-β, (D) MMP2 and MMP9 levels, (E, F) expression of TGF-β, p-Smad2/3 and Smad2/3, activities of (G) MDA, (H) GSH, (I) SOD, and (J, K) ROS production were detected in HK-2 cells treated with Ang II (1 µM), different concentrations of BSHX (0.1, 0.2, and 0.5 mg/mL), and 100 µM NAC as positive control. Data represent the mean ± SD (n = 3). One-way ANOVA followed by Dunnett’s multiple comparisons test was used. ***p < 0.001 vs. Vehicle group. !p < 0.05, !!p < 0.01, !!!p < 0.001 vs. the Ang II group. Ang II, angiotensin II; Hpy, hydroxyproline; TGF-β, transforming growth factor-beta; MMP2, matrix metalloproteinase 2; MMP9: matrix metalloproteinase 9; MDA, malondialdehyde; GSH, glutathione; SOD, superoxide dismutase; ROS, reactive oxygen species; DCFH-DA, 2’,7'-dichlorodihydrofluorescein diacetate; NAC, N-acetylcysteine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
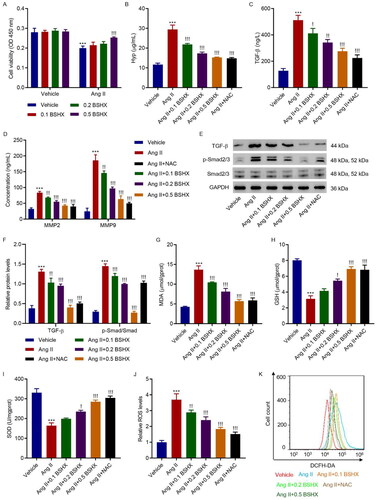
Figure 5. BSHX alleviates Ang II-induced NLRP3 inflammasome activation and pyroptosis in HK-2 cells. (A, B) The protein levels of NLRP3, caspase-1 p20, and GSDMD-N, (C) concentration of IL-1β and IL-18, and (D, E) cell pyroptosis were detected in HK-2 cells treated with Ang II (1 µM), different concentrations of BSHX (0.1, 0.2, and 0.5 mg/mL), and 100 µM NAC as positive control. Data represent the mean ± SD (n = 3). One-way ANOVA followed by Dunnett’s test for multiple comparisons was used. ***p < 0.001 vs. Vehicle group. !p < 0.05, !!p < 0.01, !!!p < 0.001 vs. the Ang II group. NLRP3, NOD-like receptor protein 3; GSDMD-N, gasdermin D-N-terminal domain; IL-1β, interleukin-1beta; IL-18, interleukin-18; PI, propidium iodide; NAC, N-acetylcysteine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
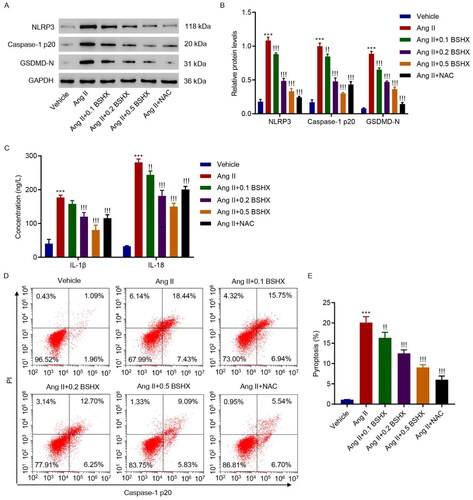
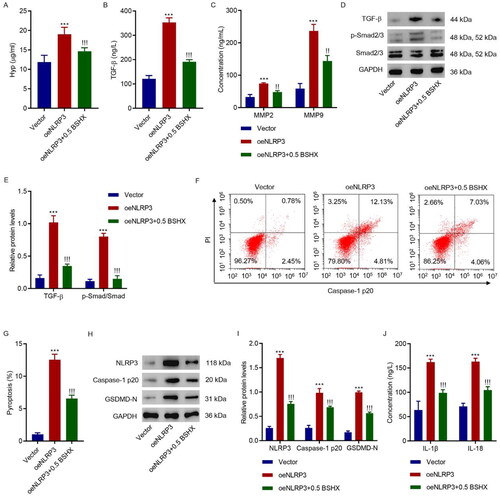
Figure 6. BSHX suppresses NLRP3-mediated fibrosis and pyroptosis in HK-2 cells. (A) Hyp, (B) TGF-β, (C) MMP2 and MMP9 levels, (D, E) expression of TGF-β, p-Smad2/3, and Smad2/3, (F, G) cell pyroptosis, (H, I) the protein levels of NLRP3, caspase-1 p20, and GSDMD-N, and (J) concentration of IL-1β and IL-18 in HK-2 cells transduced with NLRP3 overexpressing lentivirus in the presence of 0.5 mg/mL BSHX. Data represent the mean ± SD (n = 3). One-way ANOVA followed by Dunnett’s test for multiple comparisons was used. ***p < 0.001 vs. Vector group. !!!p < 0.001 vs. the oeNLRP3 group. Hpy, hydroxyproline; TGF-β, transforming growth factor-beta; MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; NLRP3, NOD-like receptor protein 3; GSDMD-N, gasdermin D-N-terminal domain; IL-1β, interleukin-1beta; IL-18, interleukin-18; PI, propidium iodide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Supplemental Material
Download TIFF Image (238.9 KB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.