Figures & data
Figure 1. Flow diagram of the implementation sequence. PFP: plasma-free plasmapheresis using a 4% human albumin solution; MICM: bone marrow morphology, immunology, cytogenetics, and molecular biology.
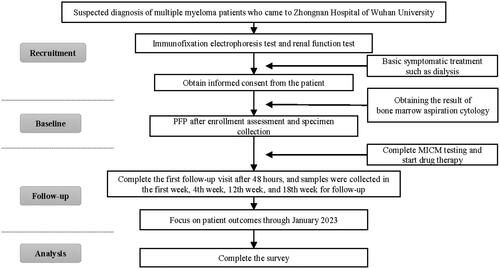
Table 1. Clinical characteristics of the primary analysis population.
Figure 2. Effects of PFP on the clearance of substances with different molecular weights. Box plots comparing the curative effects of renal biomarkers (A, B) and immunological biomarkers (C–F), demonstrating the ability of plasma-free plasmapheresis with 4% albumin (PFP) to scavenge small, medium, and large molecules. A paired t-test was performed with the values before (red) and after (blue) PFP. Green: normal reference range. The analysis results are shown as a box plot for the Durie–Salmon stage. Box: interquartile range (IQR) from the first to the third quartiles, with the median value in the middle. Whiskers: 5th to 95th percentiles. Circles: values outside this range.
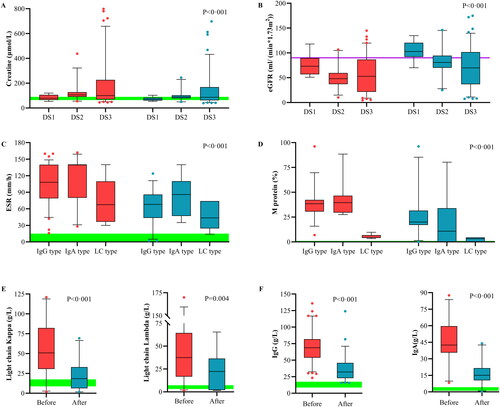
Figure 3. Outcome measures at the 18-week follow-up. Follow-up testing of renal (A, B) and immunological biomarkers (C–F). The points on the polyline represent the median value. The whiskers represent the ranges of the maximum and minimum values. The green shading represents the normal reference range. Normally, the M protein is not visible. According to the initial renal function, the patients were divided into groups A and B according to a cutoff of 20 mL/(min·1.73 m2). The light chain (LC) and Ig types were determined using immunofixation electrophoresis at enrollment. The results of chemotherapy alone and therapeutic plasma exchange (TPE) were obtained from previous studies that included patients with the same baseline estimated glomerular filtration rate (eGFR) or chemotherapy plan.
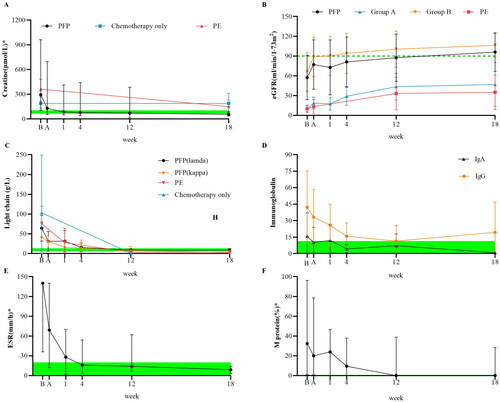
Figure 4. Effect on survival rate. K–M survival curves for enrolled patients according to free light chain (LC) type.
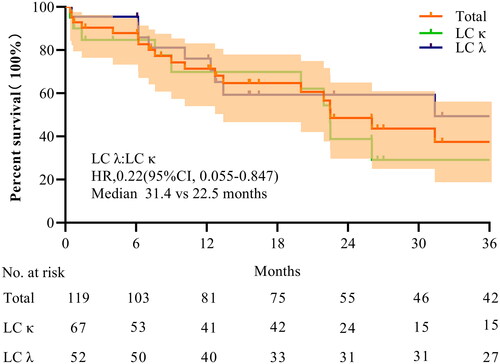
Figure 5. Subjective feelings and the first hospitalization length of stay (FLOS). The statistical results for symptom improvement (A) are based on questionnaires and nursing records. Red: significant improvement, orange: mild improvement, blue: partial improvement, and grey: no relevant symptoms (NRS) at the time of onset. The violin plot (B) shows the FLOS of multiple myeloma hospitalizations for patients with different admission statuses. According to the estimated glomerular filtration rate (eGFR) at enrollment, group A included patients with an eGFR ≤20 mL/(min·1.73 m2), whereas group B included the remaining patients. Trends in overall survival and different subgroups are shown. Patients with severe infection, anuria, systemic failure, or rheumatic disease were considered critically ill, whereas the remaining patients were considered mildly ill. The horizontal black line in the middle of the violin represents the median value. The horizontal white lines below and above represent the IQRs from the first to the third quartiles.
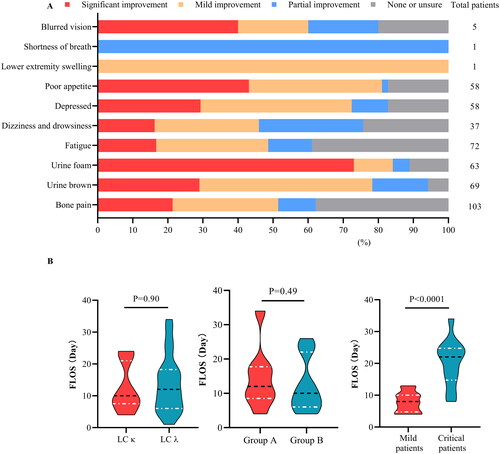
Figure 6. Variability of critical blood components. The variability of major blood components was analyzed for plasma-free plasmapheresis with 4% albumin (PFP) using box plots for blood cells (A–C), colloid components (D), and crystal components (E, F). Red: values before PFP; blue: values after PFP; green: reference values. Analysis was performed using a paired t-test, and the data are presented as a box plot for the Durie–Salmon stage. Box: interquartile range (IQR) from the first to third quartiles, with the median value in the middle. Whiskers: 5th to 95th percentiles. Circles: values outside this range.
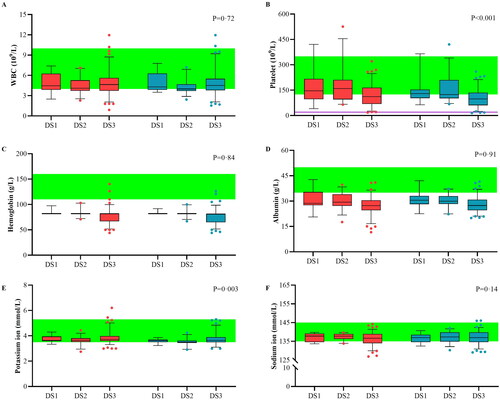
Table 2. Reported adverse events.
Data availability statement
The datasets and resources analyzed during the current study are available from the corresponding author upon reasonable request.