Figures & data
Figure 1 Immunohistochemical staining of LTA in TCRα− / − × AIM− / − mouse liver. (a) At a lower magnification, LTA immunoreactivity was prominent in the portal areas, but not in hepatic sinusoidal cells. (b) In the same sample, at a higher magnification of the portal area, LTA immunoreactivity was observed in the cytoplasm of polymorphic inflammatory cells. H&E staining of the same bile duct is shown in the right lower corner. (c) In another TCRα− / − × AIM− / − mouse liver, LTA was detected in the cytoplasm of bile duct epithelial cells (arrowheads), and connective tissue of Glisson's sheath. (d) The left lower corner shows H&E staining of the portal tract. Red blood cells and white blood cells can be observed in the portal vein branch. (P: portal vein). Amorphous materials were intermingled with blood cells in the lumens of the portal vein branch (arrows). (e) In the hepatic lobules, LTA was mainly located in the hepatocytes adjacent to the central vein. (C: central vein, P: portal vein). (f) In the same sample, at a higher magnification of the hepatic lobules, LTA localizations were clearly observed each of the single hepatocytes (C: central vein). (g) LTA immunoreactivity was not detectable in wild-type (C57BL/6J) mice liver.
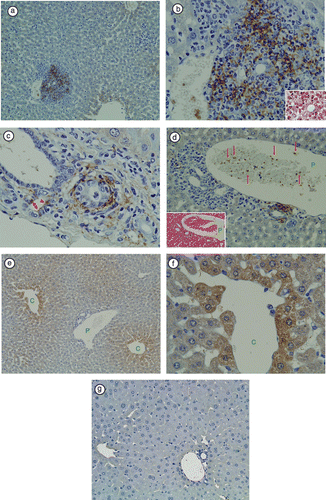
Figure 2 Pathological findings and immunohistochemical staining of LTA in the gastrointestinal tract of TCRα− / − × AIM− / − mice. (a) In the stomach, mild polymorphic inflammatory cellular infiltrates were observed in both mucosal and submucosal layers in all of the TCRα− / − × AIM− / − mice. LTA immunoreactivity was detectable in the proper mucosal layer. LTA was localized in the cytoplasm of both the fundic gland epithelial cells at the basal regions and polymorphic inflammatory cells. In the submucosal layer, immunoreactivity to LTA was observed in the cytoplasm of inflammatory cells and the stromal connective tissues. (b) At a higher magnification, LTA was localized in the cytoplasm of both the fundic gland epithelial cells at the basal regions. (c) In the small intestines of TCRα− / − × AIM− / − mice, mild to moderate polymorphic inflammatory cellular infiltrates were observed in the mucosal layers (H&E, on the higher right corner). In the proper mucosal layer, LTA immunoreactivity was located in the cytoplasm of inflammatory cells at the basal lesions. (d) In another TCRα− / − × AIM− / − mouse, mild to moderate polymorphic inflammatory cellular infiltrates were observed in the mucosal layers of the small intestine (H&E, on the lower left corner). In the proper mucosal layer, LTA immunoreactivity was located in the cytoplasm of inflammatory cells at the basal lesions. (e) In the submucosal layer, LTA immunoreactivity was observed in the connective tissue and in the cytoplasm of some of the inflammatory cells (arrow) in Peyer's patches. (f) In the colon, LTA immunoreactivity was detectable in the colon of all TCRα− / − × AIM− / − mice. LTA was detected in the cytoplasm of mucosal inflammatory cells and in the connective tissue of the proper mucosal layer.
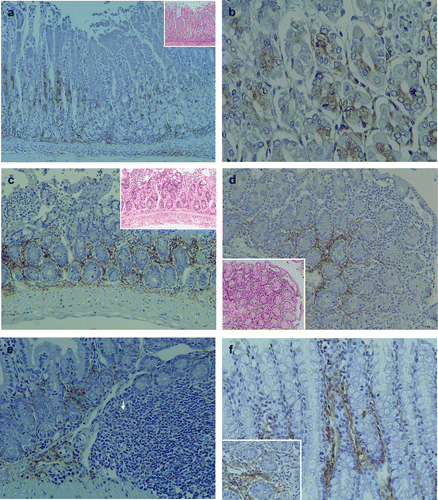
Figure 3 Pathological findings and immunohistochemical staining of LTA in the pancreas, kidney and spleen. (a) In the pancreas, marked inflammatory cellular infiltrates around pancreatic ducts were observed (H&E, lower middle column). LTA was located in the cytoplasm of polymorphic inflammatory cells and in the stromal connective tissue around pancreatic ducts (lower magnification in the left lower corner). (b) In another TCRα− / − × AIM− / − mouse, marked inflammatory cellular infiltrates around pancreatic ducts were observed (H&E, lower left corner). LTA was also detected in the cytoplasm of polymorphic inflammatory cells and the stromal connective tissue around pancreatic ducts (immunohistochemistry). (c) In the kidney, mild inflammatory cellular infiltrates around renal tubuli were observed (H&E, lower left corner). LTA immunoreactivity was detected in the cytoplasm of polymorphic inflammatory cells, and in the stromal connective tissues of the cortex. (d) In another TCRα− / − × AIM− / − mouse, LTA immunoreactivity was detected in the cytoplasm of some distal renal tubuli. Lower half shows H&E staining of the sequential sliced section. (e) LTA immunoreactivity was mainly detected in the cytoplasm of lymphocytes in the white pulp of the spleen. Lower left corner shows H&E staining. (f) In the same sample as (e), at a higher magnification of the spleen, LTA immunoreactivity was mainly detected in the cytoplasm of lymphocytes in the white pulp.
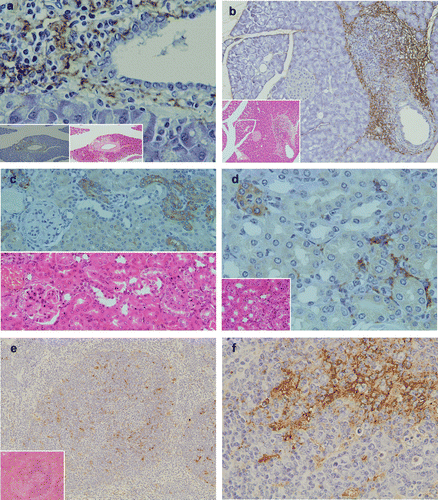
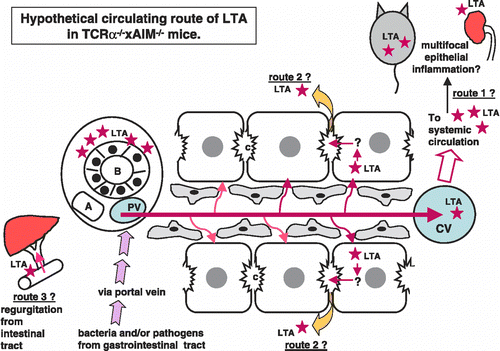
Figure 4 Hypothetical circulating route of LTA in TCRα− / − × AIM− / − mice. At first, bacteria and/or pathogens including LTA were taken from the epithelia of the gastrointestinal tract. LTA and/or bacterial components, as well as other pathogens, reached the liver via portal blood flow. These LTA and/or LTA-containing materials would be drained toward the central vein in the liver. LTA immunoreactivities were detectable not only in the liver and gastrointestinal tract, but also in the pancreas, kidney, and spleen. Via systemic blood flow, LTA could be trapped in other organs, which might possess a higher affinity to LTA (speculative route 1). LTA was also detected within the cytoplasm of hepatocytes. Perhaps LTA that escaped to be phagocitized by Kupffer cells is taken into hepatocytes via sinusoidal blood flow from the space of Disse (speculative route 2). There are three possible explanations for how LTA accumulates around the interlobular bile duct. One speculation is that from the hepatocytes, via bile canaliculi to Herring's canal, LTA was secreted and finally reached the interlobular bile ducts. The other speculation is that, because of regurgitation from intestinal tract to bile ducts, LTA finally reached the interlobular bile ducts (speculative route 3). The third speculation is that, maybe because of the high affinity of bile ducts to LTA, LTA accumulates around bile ducts via systemic arterial blood flow throughout route 1. After reaching the bile ducts, leakage of LTA around bile ducts would occur.