Figures & data
Table 1. Study populations.
Figure 1. Verification of molecular weight and protein sizes by SDS-PAGE electrophoresis and radio autography. From left to right: (A) 125I-Hypocretin 2 (2.89 kDa), 125I-Hypocretin 1 (3.58 kDa), 35S-POMC (27.25 kDa), 35S-TRIB2 (38.8 kDa); (B) 35S-KIR4.1 (42.51 kDa), 35S-HCTR2 (32.66 kDa), 35S-ppHypocretin (13.37 kDa); (C) 35S-ANO2 (95.13 kDa), and 35S- DP1 (40.3 kDa).
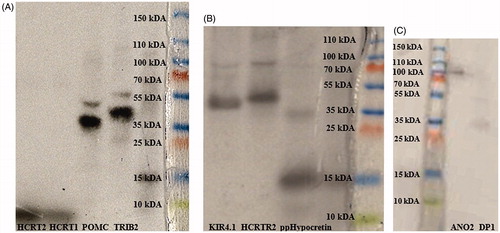
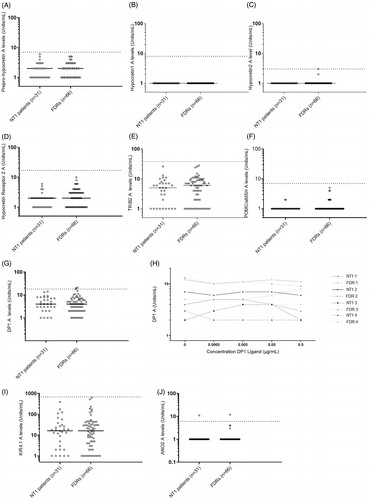
Figure 2. Autoantibodies to putative NT1 autoantigens. Comparison between narcolepsy patients and their first-degree relatives. Analyses of serum autoantibodies in narcolepsy patients (n = 31) and first-degree relative healthy family members (n = 66). Horizontal lines indicate median levels of autoantibodies and the dotted lines indicate cut-off level based on the analysis of serum samples from healthy blood donors, samples ascertained in 2008 (n = 200). Each dot represents one study participant. Subjects with autoantibody levels above the dotted line are considered positive. (A) Median levels of ppHypocretin autoantibodies were 2 (1–6) U/ml for NT1 patients and 2 (1–5) U/ml for controls in the family study (p = .576). No NT1 patients or FDR were considered positive for ppHypocretin autoantibodies. (B) Median autoantibody levels towards HCRT1 were 1 U/ml for both the NT1 patients and FDRs (p = 1). No NT1 patients or FDR were considered positive for HCRT1 autoantibodies. (C) Median levels of HCRT2 autoantibodies were low and did not differ between patients 1 (1) U/ml and FDRs 1 (1–3) U/ml (p = .33). No NT1 patients or FDR were considered positive for HCRT2 autoantibodies. (D) Median levels of HCRTR2 autoantibodies for NT1 patients were 2 (1–6) U/ml compared to 2 (1–10) U/mL for FDRs (p = .145). No NT1 patients or FDR were considered positive for HCRTR2 autoantibodies. (E) Median levels of TRIB2 autoantibodies were 5 (1–26) U/ml among NT1 patients compared to 6 (1–28) U/ml among FDRs (p = .286). Both NT1 patients and FDR were considered negative for TRIB2 autoantibodies. (F) The median levels for POMC/α-MSH autoantibodies were comparable between NT1 patients 1 (1–2) U/ml and FDRs 1 (1–5) U/ml (p = .371). Both NT1 patients and FDR were considered negative for POMC/α-MSH autoantibodies. (G) Median autoantibody levels for DP1 were comparable between patients 4 (1–14) U/ml and FDRs 4 (1–21) U/ml (p = .782). Two FDRs were considered positive for DP1 autoantibodies. (H) DP1 autoantibody levels were found to be consistent in both patients and FDRs, and not dependent on concentrations of DP1-ligand. (I) Median KIR4.1 autoantibody levels were 16 U/ml (1–391) U/ml for NT1 patients and 16 U/ml (1–622) U/ml for FDRs (p = .804). One patient and one FDR, not belonging to the same family, were considered positive for ANO2 autoantibodies. (J) ANO2 autoantibody levels were comparable between patients 1 (1–11) U/ml and FDRs 1 (1–12) U/ml (p = .572). Both patients and FDRs were negative for KIR4.1 autoimmunity.