Figures & data
Figure 1. The lncRNA RP5-857K21.7 is significantly downregulated while miR-508-3p upregulated in PDGF-BB-induced ASMCs. (A) QRT-PCR analysis shows that the stimulation of ASMCs with PDGF-BB significantly inhibited RP5-857K21.7 mRNA expression level compared to the blank control group, suggesting that RP5-857K21.7 expression could regulate the biological effect of PDGF-BB on ASMCs. (B) QRT-PCR analysis confirmed that miR-508-3p was markedly upregulated in PDGF-BB-induced ASMCs, indicating that abnormal miR-508-3p expression may play a role in PDGF-BB-induced ASMCs. All experimental data are shown as the mean ± SD of at least three independent experiments with a significance level of p < .05 (**p < .01).
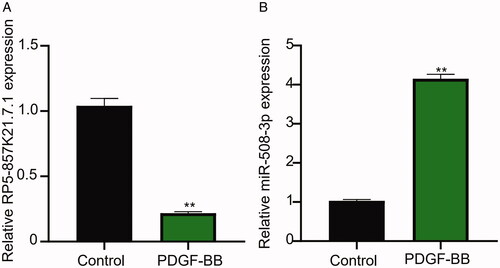
Figure 2. The overexpression of RP5-857K21.7 inhibits PDGF-BB-induced proliferation and migration of ASMCs and promotes its apoptosis (A) The efficacy of the constructed RP5-857K21.7 overexpression plasmid confirmed through qRT-PCR analysis. RP5-857K21.7 mRNA expression was significantly upregulated in the cells transfected with the RP5-857K21.7 plasmid vector compared to those transfected with an empty vector. (B) CCK8 assay revealed a significant increase in the proliferation of ASMCs after induction with PDGF-BB compared to the control group while the overexpression of RP5-857K21 significantly reduced the proliferative ability of the PDGF-BB-induced ASMCs when compared to the PDGF-BB + vector group. (C) Transwell assay showed that PDGF-BB-induction significantly increased the migration of the ASMCs compared to the control group, while RP5-857K21.7 overexpression notably reduced the migrative ability of the cell. (D) Flow cytometry assay showed that the rate of apoptosis in the PDGF-BB-induced ASMCs was higher after overexpression with RP5-857K21 compared to the empty vector group. All experiments were conducted in triplicates and the experimental data are presented as the mean ± SD of the experiments with the significance level defined as p < .05.
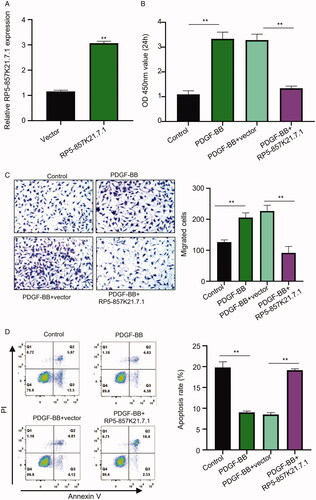
Figure 3. RP5-857K21.7 directly targets miR-508-3p (A) Bioinformatics prediction of RP5-857K21.7 target miRNA using miRcode online database (B) Confirmation of prediction result through dual-luciferase reporter gene assay. MiR-508-3p mimics significantly inhibited the luciferase activity of cells transfected with the RP5-857K21.7 wild-type (wt) vector but had no significant inhibiting impact on the luciferase activity of ASMCs transfected with the RP5-857K21.7 mutant-type (mut) vector when compared to miR-NC. (C) QRT-PCR analysis of miR-508-3p expression in ASMCs. MiR-508-3p mimics significantly upregulated miR-508-3p expression in ASMCs compared to miR-NC. (D) Biotinylated RNA pull-down assay showed that more miR-508-3p was significantly enriched in the ASMCs transfected with the biotin-labelled RP5-857K21.7 wild-type (WT) probe compared to those transfected with the mutant-type (MUT). RP5-857K21.7 can directly interact with miR-508-3p sequence in in ASMCs cells (E) RIP-qRT-PCR revealed that more RP5-857K21.7 and miR-508-3p sequence were found to be significantly enriched in precipitated Ago2 compared to the IgG. (F) QRT-PCR indicated that the overexpression of RP5-857K21.7 significantly increased the expression level of miR-508-3p in ASMCs, validating the regulative ability of RP5-857K21.7. The experimental data are presented as the mean ± SD of at least three independent experiments and the significance level is defined as p < .05.
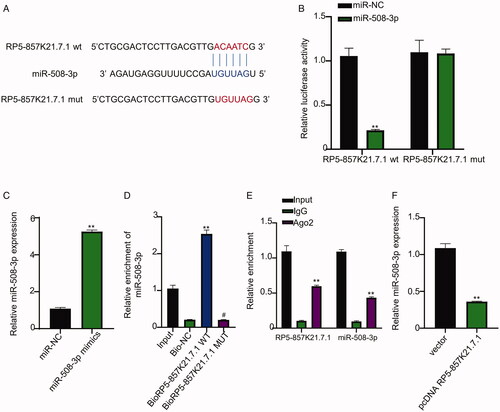
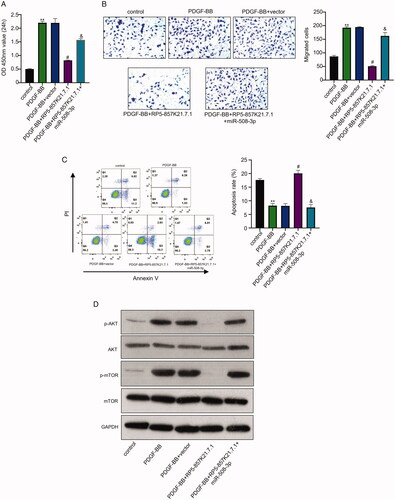
Figure 4. The RP5-857K21.7 regulates the PI3K/AKT/mTOR pathway by sponging miR-508-3p in ASMCs cells (A) and (B) CCK-8 and transwell assay shows that the overexpression of RP5-857K21.7 markedly reduced the proliferation and migration of the PDGF-BB-induced ASMCs compared to the empty vector while the co-transfection of miR-508-3p mimics significantly restored the cell proliferation and migration. (C) Flow cytometry detection of apoptosis rate of PDGF-BB-induced ASMCs showed that overexpressing RP5-857K21.7 significantly increased the rate of apoptosis in the cell which was then reduced after co-transfection with miR-508-3p mimics. (D) Western blot analysis revealed that the PI3K and p-mTOR protein expression level markedly increased in the ASMCs after PDGF-BB induction compared to the control group while that of AKT and mTOR protein remain unchanged. Data are presented as the mean ± SD of at least three independent experiments with a significance level of p < .05.
Data availability statement
All data generated or analysed during this study are included in this published article and its additional files.
