Figures & data
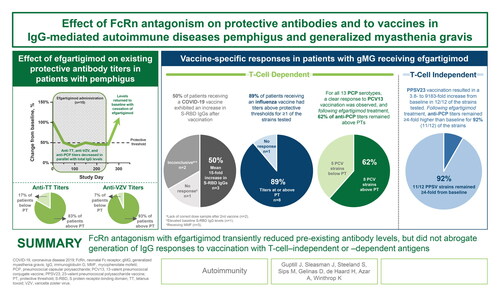
Figure 1. Schematic illustration of pemphigus and gMG trials, trial dosing, and post-hoc analysis conducted.
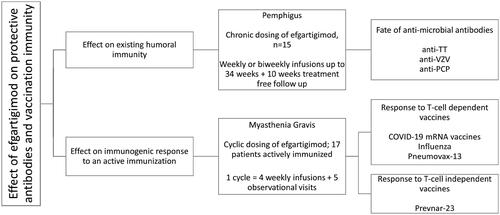
Figure 2. (A) Mean percent change from baseline for anti-varicella zoster virus (VZV), anti-tetanus toxin (TT), anti–pneumococcal capsular polysaccharide (PCP), anti-desmoglein 1 (Dsg1), anti-desmoglein 3 (Dsg3), and total immunoglobulin G (tIgG) antibodies in 15 patients treated for up to 238 days (34 weeks) with efgartigimod. For anti-Dsg 1/3 antibodies, data is plotted for patients who presented with positive anti-Dsg1/3 autoantibody levels ≥ 20 U/mL at baseline (n = 13 for anti-Dsg1, n = 8 for anti-Dsg3), excluding one patient who presented with positive anti-Dsg3 autoantibody levels only on day 235 when relapse occured. Error bars represent standard error of the mean (SEM). (B) Percent change from baseline for anti-VZV and tIgG in a patient with pemphigus vulgaris (PV) exhibiting a transient increase in anti-VZV IgG titres. Treatment period is indicated by background shading.
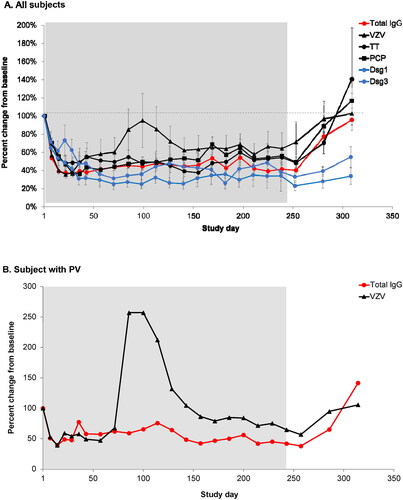
Figure 3. Levels of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S protein receptor-binding domain (S-RBD) immunoglobulin G (IgG) before the first and after the second dose (Day 1) of BNT162b2 (A-F) or mRNA-1273 (G) in individual patients with generalised myasthenia gravis (gMG) who participated in ADAPT+. Shaded areas represent efgartigimod treatment cycles. The dashed line represents the lower limit of quantitation. The lowest total IgG levels are expected 1 week after the last efgartigimod infusion of each cycle (red arrows).
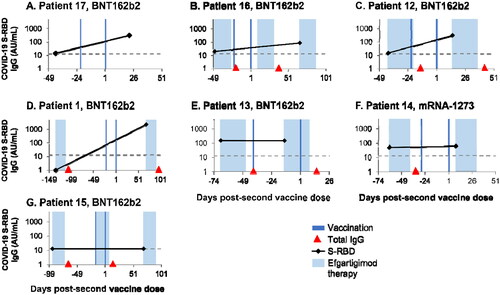
Figure 4. . Individual hemagglutination inhibition (HI) titers from 7 patients with generalized myasthenia gravis (gMG) vaccinated against influenza during the double-blind treatment period or open-label extension of ADAPT. (A) Patients who received only placebo. (B) Patients treated with efgartigimod and vaccinated between treatment cycles. (C) Patients treated with efgartigimod and vaccinated within a treatment cycle. (D) Efgartigimod-treated patient who was vaccinated but infected with influenza. PT, protection threshold; ULQ, upper limit of quantification. Solid black line indicates day of vaccination. Dotted black line indicates date of influenza infection.
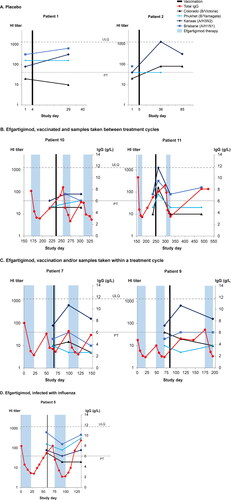
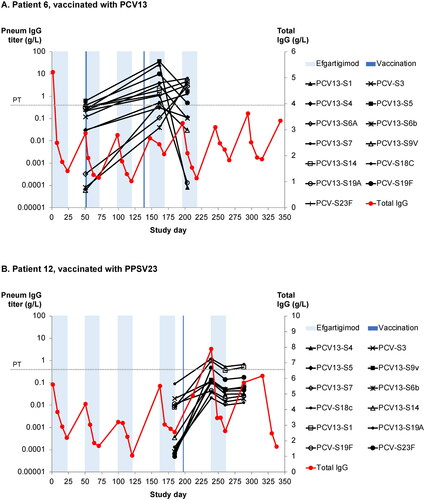
Figure 5. (A) Anti–pneumococcal capsular polysaccharide (PCP) titres against 13 PCP serotypes in an efgartigimod-treated patient after vaccination with 13-valent pneumococcal conjugate vaccine (PCV13). The protective threshold protecting against invasive pneumococcal infection is 350 ng/mL (dotted line). (B) Anti-PCP titres against 12 serotypes in an efgartigimod-treated patient after vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV13). Shaded areas represent efgartigimod treatment periods.
Supplemental Material
Download MS Word (164.4 KB)Data availability statement
argenx is committed to responsible data sharing regarding the clinical trials they fund. Included in this commitment is access to anonymised, individual, and trial-level data (analysis datasets), and other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by qualified researchers who engage in rigorous independent scientific research and will only be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months. Requests can be submitted to [email protected].
