Figures & data
Figure 1. Apoptosis in protozoan infection. (a) Parasite infection. Protozoan parasites invade and multiply within host cells. Inhibition of host cell apoptosis by parasites contributes to the establishment of infection. (b) Cell-mediated immunity. CD8 T cells releasing perforin- and GrB-containing granules, and CD4 T cells expressing FasL induce apoptosis of infected cells. In addition, both CD4 and CD8 T cells secrete IFN-γ and activate macrophages to produce NO, which kills parasites. Cytokines TGF-β and IL-10 antagonize IFN-γ effects on macrophages and favor infection. (c) T-cell apoptosis. Activated CD4 and CD8 T cells express Fas and FasL and undergo activation-induced cell death. Either the disappearance of T cells or the effect of apoptotic cells on macrophages result in defective immune responses. Apoptotic cells induce macrophages to produce TGF-β, which increases parasite replication within infected macrophages. (d) Tissue lesion. CD8 T cells and CD4 T cells infiltrate tissues and kill both infected and non infected cells, causing tissue dysfunction.
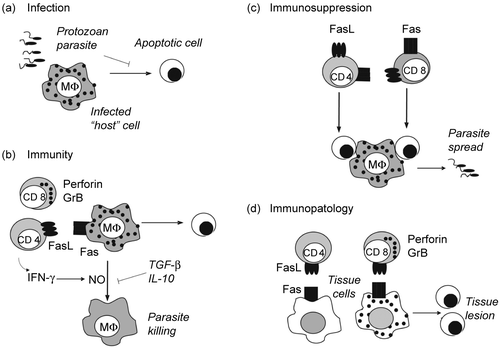
Figure 2. Molecular targets for inhibition of cell death signaling. (a) Extrinsic pathway. FasL induces apoptosis upon interaction with death receptor Fas, followed by recruitment of the adapter protein FADD and caspase-8 to the death inducing signaling complex (DISC). Caspase-8 activation depends on caspase-8 dimerization and is inhibited by heterodimer formation with cFLIP. (b) Cytotoxic pathway. Cytotoxic T cells (CTL) induce apoptosis by releasing granules containing Granzyme B (GrB) and perforin. Perforin promotes release of GrB to the cytoplasm, where GrB activates caspases. (c) Intrinsic pathway. Growth factor deprivation changes the ratio between pro and anti- apoptotic members of Bcl-2 family. Bim activates Bax, promoting the release of cytochrome c from mitochondria. Growth factors induce the expression of antiapoptotic Bcl-2 family members (Bcl-2 and Mcl-1), which sequester Bim and prevent cytochrome c release and apoptosis. Cytochrome c, APAF-1, and caspase-9 form the apoptosome in the cytoplasm, where caspase-9 becomes active. (a–c) The initiator caspases 8 and 9 or GrB cleave and activate effector caspases 3, 6 and 7. Apoptosis is induced by the action of effector caspases on cell substrates. Inhibitor of apoptosis proteins (IAPs) inhibit both caspase-9 and caspase-3. Potential targets for inhibition of apoptosis and caspase signaling were identified by numbers within parentheses (1–7).
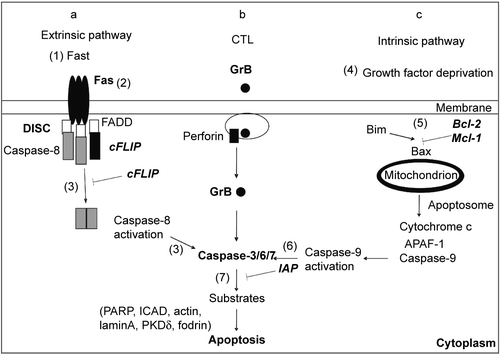
Table 1. Pro- and antiapoptotic mechanism.
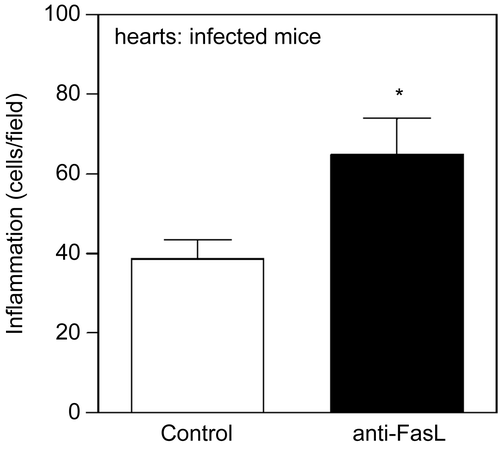
Figure 3. Treatment with anti-FasL increases inflammation in the hearts of T. cruzi- infected mice. BALB/c mice were infected with T. cruzi and received two injection of anti-FasL (0.15 mg/mouse). Control group received either saline or a control hamster IgG antibody.(Citation78) Hearts were collected 25 days post infection and analyzed for inflammatory infiltrates in slides stained with Hematoxilin and eosin. Results were expressed as number of cells/microscopic field. The number of cells/field in the normal mouse heart (6.4) was deducted from the results for each infected mouse. Significant difference is indicated (*) for p< 0.05, n = 4 mice/group.