Figures & data
Figure 1. GC–MS chromatogram of antibiofilm methanol extract of S. persica showing the compounds and their percentage abundance (parenthesis) 1. benzaldehyde (13.2); 2. benzyl chloride (1.6); 3. ethanone, 2-hydroxy-1-phenyl-(0.09); 4. benzyl isocyanate (7.2); 5. benzyl nitrile (2.0); 6. benzenecarboxylic acid (24.5); 7. l-[-]-4-hydroxy-1-methylproline(0.1); 8. benzaldehyde, 4-methoxy-(0.1); 9. benzaldehyde, 3-methoxy-(0.5); 10. 2-coumaranone (0.4); 11. benzeneacetic acid (0.07); 12. 3-benzyloxy-1-nitro-butan-2-Ol (0.01); 13. benzaldehyde, 4-(phenylmethoxy)- (0.3); 14. benzene isothiocyanate (18.1); 15. 2-(3’-phenylpropyl)-5-ethylpyridine (0.8); 16. (Z,Z)-à-farnesene (0.3); 17. N- benzylacetamide (10.9); 18. benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate (0.02); 19. 1,3-cyclohexanedicarbohydrazide (0.01); 20. benzylidenebenzylamine (12.1); 21. 3à-hydroxy-3-methyl-6-phenyl-4-piperidone (0.8); 22. decanoic acid, methyl ester (0.1); 23. docosanoic acid, ethyl ester (0.08); 24. acetophenone benzyloxime (0.43); 25. benzamide, N-(4-methylphenyl)- (0.45); 26. pyrrole-2-carboxylic acid, 4-hydrazonomethyl-3,5-dimethyl-, ethyl ester(0.3).
![Figure 1. GC–MS chromatogram of antibiofilm methanol extract of S. persica showing the compounds and their percentage abundance (parenthesis) 1. benzaldehyde (13.2); 2. benzyl chloride (1.6); 3. ethanone, 2-hydroxy-1-phenyl-(0.09); 4. benzyl isocyanate (7.2); 5. benzyl nitrile (2.0); 6. benzenecarboxylic acid (24.5); 7. l-[-]-4-hydroxy-1-methylproline(0.1); 8. benzaldehyde, 4-methoxy-(0.1); 9. benzaldehyde, 3-methoxy-(0.5); 10. 2-coumaranone (0.4); 11. benzeneacetic acid (0.07); 12. 3-benzyloxy-1-nitro-butan-2-Ol (0.01); 13. benzaldehyde, 4-(phenylmethoxy)- (0.3); 14. benzene isothiocyanate (18.1); 15. 2-(3’-phenylpropyl)-5-ethylpyridine (0.8); 16. (Z,Z)-à-farnesene (0.3); 17. N- benzylacetamide (10.9); 18. benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate (0.02); 19. 1,3-cyclohexanedicarbohydrazide (0.01); 20. benzylidenebenzylamine (12.1); 21. 3à-hydroxy-3-methyl-6-phenyl-4-piperidone (0.8); 22. decanoic acid, methyl ester (0.1); 23. docosanoic acid, ethyl ester (0.08); 24. acetophenone benzyloxime (0.43); 25. benzamide, N-(4-methylphenyl)- (0.45); 26. pyrrole-2-carboxylic acid, 4-hydrazonomethyl-3,5-dimethyl-, ethyl ester(0.3).](/cms/asset/3883b89e-da7e-466c-9ccb-424278c6c6cd/gbif_a_647308_o_f0001g.jpg)
Figure 2. Crystal structure of quorum sensing response regulator (PDB ID: INXO) (R) and its super imposed structures of (a) benzaldehyde, 4-methoxy-; (b) benzaldehyde, 3-methoxy-; (c) benzeneacetic acid; (d) benzyloxy-1-nitro-butan-2-ol; (e) benzaldehyde, 4-(phenylmethoxy)-; (f,g) N-benzylacetamide; (h) 1,3-cyclohexane dicarbo hydrazide; (i) decanoic acid, methyl ester; (j) docosanoic acid, ethyl ester; (k) benzamide, N-(4-methylphenyl)-; (l) benzyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate; (m) benzyl isocyanate; (n) acetophenone benzyloxime; (o) chlorohexidine. The illustration demonstrates close alignment of molecular components of S.persica phytochemicals and the QS protein. The antibiotic chlorhexidine (2o) also interacts in the same manner as most of the phytochemicals.
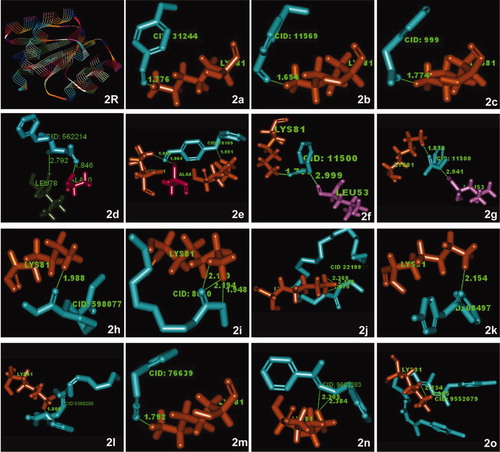
Table 1. Growth inhibition and biofilm inhibiting activity of S. persica extracts on caries causing S. mutans.
Table 2. Ligandfit docking scores of the bioactive compounds of S. persica and the antibiotic chlorhexidine with the active site of the DNA-binding response regulator (PDB ID: 1NXO).
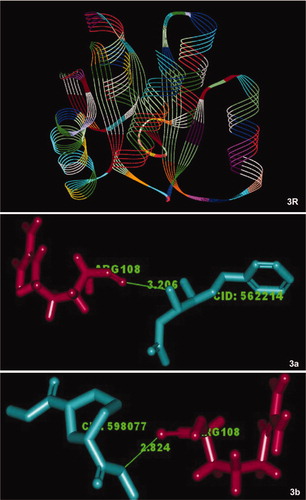
Figure 3. The DNA-binding response regulator (PDB ID: 3B2N) (R), LuxR family from S. aureus that was used for docking experiments with the bioactive compounds of S. persica 3-benzyloxy-1-nitro-butan-2-ol (a) and 1,3-cyclohexanedicarbohydrazide (b). The illustration demonstrates close alignment of the molecular components.