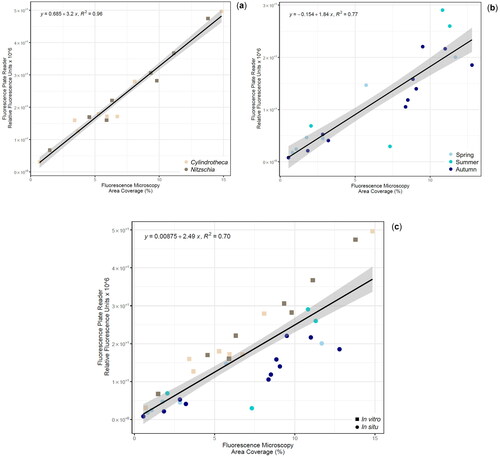
Figures & data
Figure 1. In vitro autofluorescence measurements of 9-day Cylindrotheca closterium biofilms, as recorded with (A) light (top) and fluorescence microscopy (bottom) images (scale bars = 20 μm), and (B) merged fluorescence microscopy and plate reader fluorescence records, where bars represent standard error.
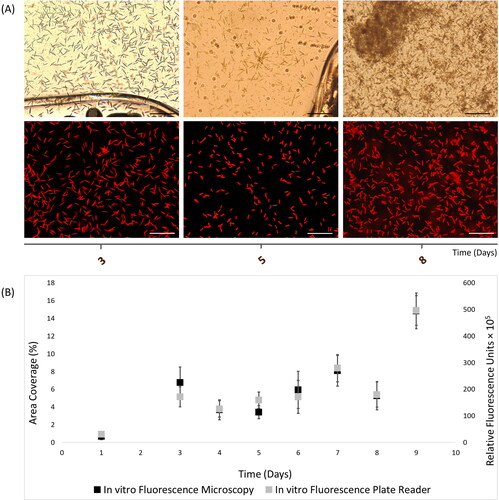
Figure 2. In vitro autofluorescence measurements of 9-day Nitzschia thermaloides biofilms, as recorded with (A) light (top) and fluorescence (bottom) microscopy images (scale bars = 20 μm), and (B) merged fluorescence microscopy and plate reader fluorescence records, where error bars represent standard error.
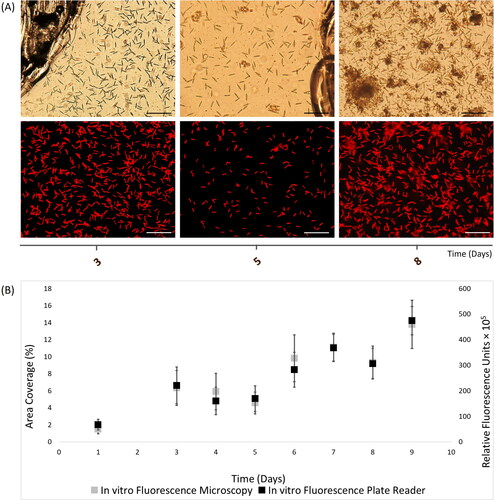
Figure 3. In situ autofluorescence measurements of 19-day natural phototrophic biofilms in Southsea Marina UK during Spring 2017, as recorded with (A) light (top) and fluorescence microscopy (bottom) images (scale bars = 20 μm), and (B) merged fluorescence microscopy and plate reader fluorescence records, where error bars represent standard error.
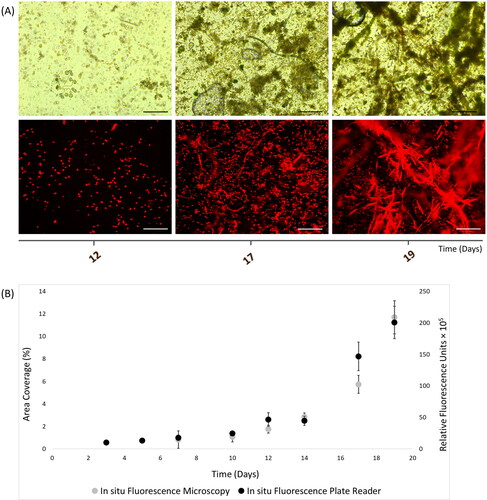
Figure 4. In situ autofluorescence measurements of 28-day natural phototrophic biofilms in Southsea Marina UK during Summer 2017, as recorded with (A) light (top) and fluorescence microscopy (bottom) images (scale bars = 20 μm), and (B) merged fluorescence microscopy and plate reader fluorescence records, where error bars represent standard error.
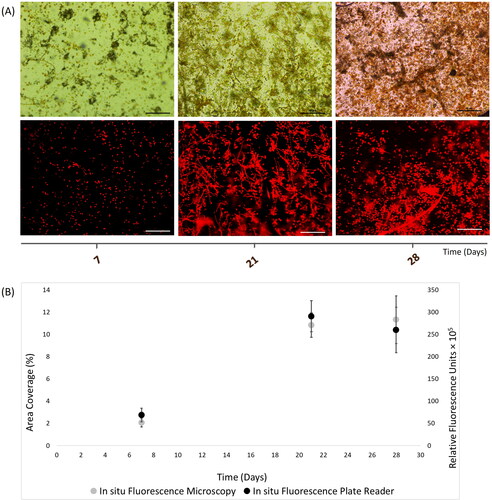
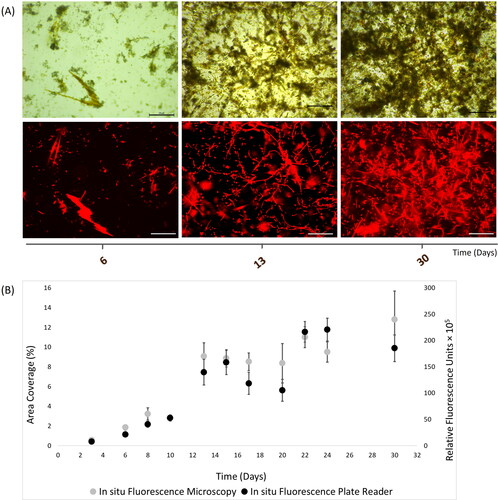
Figure 5. In situ autofluorescence measurements of 30-day natural phototrophic biofilms in Southsea Marina UK during Autumn 2017, as recorded with (A) light (top) and fluorescence microscopy (bottom) images (scale bars = 20 μm), and (B) merged fluorescence microscopy and plate reader fluorescence records, where error bars represent standard error.