Figures & data
Figure 2. (Colour online) (a) Self-diffusion coefficients of pure water at 298.15 K and 1 atm for six system sizes consisting of 512–8000 molecules. (b) Self-diffusion coefficients of binary LJ mixtures at a reduced temperature of 0.65 and a reduced pressure of 0.05 for LJ, and (c) LJ
for four system sizes consisting of 500, 1000, 2000, and 4000 particles. (d) Self-diffusion coefficients of toluene, (e) acetone and (f) water in a ternary mixture of toluene-acetone-water at 298.15 K and 1 atm (
,
, and
) for 5 system sizes consisting of 400–1500 molecules. The uncorrected MD results are shown with red circles. Grey diamonds show the corrected self-diffusion coefficients using Equation (Equation1
(1)
(1) ). Blue dashed lines are the linear extrapolation of MD results to the thermodynamic limit and black dashed lines are the extrapolated values. The axes of subfigures scales differently.
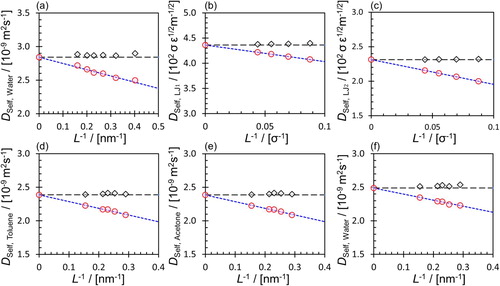
Figure 3. (Colour online) (a) MS and (b) Fick diffusion coefficients of a binary equimolar methanol–methylamine mixture at 298 K and 1 atm as a function of the simulation box length (L). (c) MS and (d) Fick diffusion coefficients of binary LJ mixtures ( and
) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS and Fick diffusion coefficients using Equations (Equation19
(19)
(19) ) and (Equation21
(21)
(21) ), respectively. Black dashed lines are the corrected diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest sizes (
and
) to the thermodynamic limit. For LJ systems, simulations were performed for four system sizes consisting of 250, 500, 1000, and 2000 particles. For the molecular mixture, four system sizes consisting of 500, 1000, 2000, and 4000 particles were considered. The results are based on the study in [Citation128]. The axes of subfigures scales differently.
![Figure 3. (Colour online) (a) MS and (b) Fick diffusion coefficients of a binary equimolar methanol–methylamine mixture at 298 K and 1 atm as a function of the simulation box length (L). (c) MS and (d) Fick diffusion coefficients of binary LJ mixtures (x1=0.3 and x2=0.7) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS and Fick diffusion coefficients using Equations (Equation19(19) ð∞=ðMD+1ΓkBTξ6πηL=ðMD+1ΓDYH(19) ) and (Equation21(21) D∞=DMD+DYH(21) ), respectively. Black dashed lines are the corrected diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest sizes (NMolecular=250 and NLJ=500) to the thermodynamic limit. For LJ systems, simulations were performed for four system sizes consisting of 250, 500, 1000, and 2000 particles. For the molecular mixture, four system sizes consisting of 500, 1000, 2000, and 4000 particles were considered. The results are based on the study in [Citation128]. The axes of subfigures scales differently.](/cms/asset/2abf98e0-a1b9-4f66-87d0-79d580c3d767/gmos_a_1810685_f0003_oc.jpg)
Figure 4. (Colour online) Fick diffusion coefficients of a ternary molecular mixture (molar reference frame) of (1) chloroform (2) acetone, and (3) methanol ( and
) at 298 K and 1 atm as a function of the simulation box length (L). (a) Diagonal component
, (b) Off-diagonal component
, (c) Off-diagonal component
, and (d) Diagonal component
. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22
(22)
(22) ). Black dashed lines are the corrected Fick diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation128]. The axes of subfigures scales differently.
![Figure 4. (Colour online) Fick diffusion coefficients of a ternary molecular mixture (molar reference frame) of (1) chloroform (2) acetone, and (3) methanol (xchloroform=xacetone=0.3 and xmethanol=0.4) at 298 K and 1 atm as a function of the simulation box length (L). (a) Diagonal component D1,1, (b) Off-diagonal component D1,2, (c) Off-diagonal component D2,1, and (d) Diagonal component D2,2. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22(22) [D∞]=[DMD]+DYH[I](22) ). Black dashed lines are the corrected Fick diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation128]. The axes of subfigures scales differently.](/cms/asset/b4e03ece-2453-4332-8f84-4b1417d1f2e5/gmos_a_1810685_f0004_oc.jpg)
Figure 5. (Colour online) MS diffusion coefficients of a ternary molecular mixture of (1) chloroform (2) acetone, and (3) methanol (, and
) at 298 K and 1 atm as a function of the simulation box length (L). (a)
, (b)
, and (c)
. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS diffusion coefficients based on Equation (Equation23
(23)
(23) ). Black dashed lines are the corrected MS diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scales differently.
![Figure 5. (Colour online) MS diffusion coefficients of a ternary molecular mixture of (1) chloroform (2) acetone, and (3) methanol (xchloroform=xacetone=0.3, and xmethanol=0.4) at 298 K and 1 atm as a function of the simulation box length (L). (a) ð1,2, (b) ð1,3, and (c) ð2,3. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS diffusion coefficients based on Equation (Equation23(23) [Δ∞]=[ΔMD]+DYH[Γ]−1(23) ). Black dashed lines are the corrected MS diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scales differently.](/cms/asset/2dfbf1dc-3e02-4390-85a6-84367677c595/gmos_a_1810685_f0005_oc.jpg)
Figure 6. (Colour online) Fick diffusion coefficients of a ternary LJ mixture (molar reference frame) ( and
) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a) Diagonal component
, (b) Off-diagonal component
, (c) Off-diagonal component
, and (d) Diagonal component
. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22
(22)
(22) ). Black dashed lines are the corrected Fick diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scale differently.
![Figure 6. (Colour online) Fick diffusion coefficients of a ternary LJ mixture (molar reference frame) (x1=0.4 and x2=x3=0.3) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a) Diagonal component D1,1, (b) Off-diagonal component D1,2, (c) Off-diagonal component D2,1, and (d) Diagonal component D2,2. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22(22) [D∞]=[DMD]+DYH[I](22) ). Black dashed lines are the corrected Fick diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scale differently.](/cms/asset/d0e98222-bd4f-4773-bed0-dc2a85678c4c/gmos_a_1810685_f0006_oc.jpg)
Figure 7. (Colour online) MS diffusion coefficients of a ternary LJ mixture ( and
) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a)
, (b)
, and (c)
. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS diffusion coefficients based on Equation (Equation23
(23)
(23) ). Black dashed lines are the corrected MS diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scales differently.
![Figure 7. (Colour online) MS diffusion coefficients of a ternary LJ mixture (x1=0.4 and x2=x3=0.3) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a) ð1,2, (b) ð1,3, and (c) ð2,3. The uncorrected MD results are shown with red circles. Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit. Grey diamonds show the corrected MS diffusion coefficients based on Equation (Equation23(23) [Δ∞]=[ΔMD]+DYH[Γ]−1(23) ). Black dashed lines are the corrected MS diffusion coefficients computed from the linear extrapolation of the Onsager coefficients of the smallest size (N=500) to the thermodynamic limit. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scales differently.](/cms/asset/8b22f683-b90f-45e8-a867-29658fb13214/gmos_a_1810685_f0007_oc.jpg)
Figure 8. (Colour online) Onsager coefficients of a ternary LJ mixture ( and
) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a)
, (b)
, (c)
, (d)
, (e)
, and (f)
. The finite-size MD results are shown with red circles. Blue dashed lines are the linear fits to the MD results. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scale differently.
![Figure 8. (Colour online) Onsager coefficients of a ternary LJ mixture (x1=0.4 and x2=x3=0.3) at a reduced temperature of 0.65 and a reduced pressure of 0.05 as a function of the simulation box length (L). (a) Λ11, (b) Λ22, (c) Λ33, (d) Λ12, (e) Λ13, and (f) Λ23. The finite-size MD results are shown with red circles. Blue dashed lines are the linear fits to the MD results. Simulations were performed for four system sizes consisting of 500, 1000, 2000, and 4000 particles. The results are based on the study in [Citation117]. The axes of subfigures scale differently.](/cms/asset/56ffaad5-bc38-4d56-be2e-926ad8a0ee20/gmos_a_1810685_f0008_oc.jpg)
Figure 9. (Colour online) Fick diffusion coefficients of an equimolar quaternary LJ mixture (molar reference frame) at a reduced temperature of 2 and a reduced pressure of 3.4 as a function of the simulation box length (L). (a) Diagonal component , (b) Off-diagonal component
, (c) Off-diagonal component
, (d) Off-diagonal component
, (e) Diagonal component
, (f) Off-diagonal component
, (g) Off-diagonal component
, (h) Off-diagonal component
, and (i) Diagonal component
. The uncorrected MD results are shown with red circles. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22
(22)
(22) ). Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit and Black dashed lines are the extrapolated values. Simulations were performed for six system sizes consisting of 400, 800, 1200, 1600, 3200, and 4800 particles. The axes of subfigures scales differently.
![Figure 9. (Colour online) Fick diffusion coefficients of an equimolar quaternary LJ mixture (molar reference frame) at a reduced temperature of 2 and a reduced pressure of 3.4 as a function of the simulation box length (L). (a) Diagonal component D1,1, (b) Off-diagonal component D1,2, (c) Off-diagonal component D1,3, (d) Off-diagonal component D2,1, (e) Diagonal component D2,2, (f) Off-diagonal component D2,3, (g) Off-diagonal component D3,1, (h) Off-diagonal component D3,2, and (i) Diagonal component D3,3. The uncorrected MD results are shown with red circles. Grey diamonds show the corrected Fick diffusion coefficients using Equation (Equation22(22) [D∞]=[DMD]+DYH[I](22) ). Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit and Black dashed lines are the extrapolated values. Simulations were performed for six system sizes consisting of 400, 800, 1200, 1600, 3200, and 4800 particles. The axes of subfigures scales differently.](/cms/asset/0625d46d-d70b-4d21-a2f2-8c6cccdbdcd9/gmos_a_1810685_f0009_oc.jpg)
Figure 10. (Colour online) MS diffusion coefficients of an equimolar quaternary LJ mixture at a reduced temperature of 2 and a reduced pressure of 3.4 as a function of the simulation box length (L).(a) Ð1,2, (b) Ð1,3, (c) Ð1,4, (d) Ð2,3, (e) Ð2,4, and (f) Ð3,4. The uncorrected MD results are shown with red circles. Grey diamonds show the corrected MS diffusion coefficients using Equation (Equation22(22)
(22) ). Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit and black dashed lines are the extrapolated values. Simulations were performed for six system sizes consisting of 400, 800, 1200, 1600, 3200, and 4800 particles. The axes of subfigures scales differently.
![Figure 10. (Colour online) MS diffusion coefficients of an equimolar quaternary LJ mixture at a reduced temperature of 2 and a reduced pressure of 3.4 as a function of the simulation box length (L).(a) Ð1,2, (b) Ð1,3, (c) Ð1,4, (d) Ð2,3, (e) Ð2,4, and (f) Ð3,4. The uncorrected MD results are shown with red circles. Grey diamonds show the corrected MS diffusion coefficients using Equation (Equation22(22) [D∞]=[DMD]+DYH[I](22) ). Blue dashed lines are the linear extrapolation of the MD results to the thermodynamic limit and black dashed lines are the extrapolated values. Simulations were performed for six system sizes consisting of 400, 800, 1200, 1600, 3200, and 4800 particles. The axes of subfigures scales differently.](/cms/asset/7502c4e8-a399-4b19-8b3c-0410f1104d6f/gmos_a_1810685_f0010_oc.jpg)