Figures & data
FIGURE 1 FLOSORB, bovine-derived gelatin particles (A, BGP), is a more course powder than ARISTA AH, microporous polysaccharide hemospheres (F, MPH). The FLOSORB granules are of irregular shape and the ARISTA AH are round (B, G, and Supplemental Figure S1). FLOSORB particles shown before (B, C) and after (D, E) hydration, reversibly swell uniformly (Supplemental Video V1). ARISTA AH particles shown before (G, H) and after (I, J) hydration swell irregularly then fuse (Supplemental Video V2). When manipulated, the FLOSORB particles remain separate (Supplemental Video V3) while the ARISTA AH particles form a cohesive sheet (Supplemental Video V4). Scale bars are 500 µm.
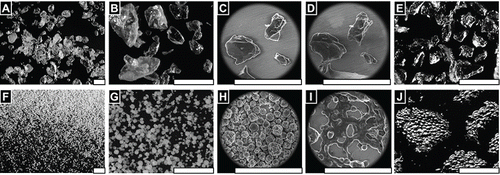
FIGURE 3 FLOSORB granules (BGP) swell uniformly when hydrated, and dehydration reveals that granules return to their original size and shape (Supplemental Video V1). In contrast, ARISTA AH particles (MPH) swell non-uniformly when hydrated, and dehydration reveals that particles fuse into aggerates with adjacent particles (Supplemental Video V5). Still images are shown before hydration ∼20%RH (A, D), at saturation >100% RH (B, C), and after dehydration ∼20% RH (C, F). Water droplets are seen condensing on the particles and stage (B, E). Scale bars are 200 µm.
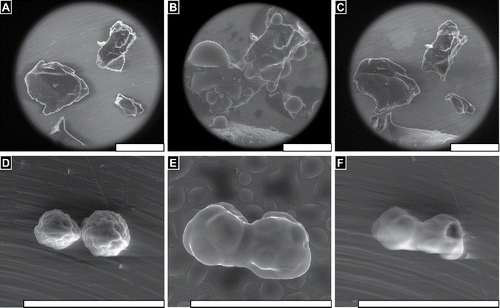
FIGURE 4 FLOSORB (BGP) provided faster and sustained hemostasis over 10 minutes relative to ARISTA AH (MPH) in a heparinized porcine hepatic bleeding model. (A, Left) Application of FLOSORB to a grade 1 bleeding site and (A, Right) an untreated grade 1 lesion (ooze or intermittent flow, estimated >1 - 5 mL/minute rate of blood loss); (B) Cessation of bleeding 2–minutes after treatment and approximation with saline-moistened gauze (FLOSORB, left; ARISTA AH, right); (C) FLOSORB-treated lesion at 10 -minutes after application (left) and a ARISTA AH-treated lesion continuing to bleed (right); (D) FLOSORB granules adhere to liver surface while (G) ARISTA AH particles leave voids between clot material and tissue surfaces. (E) FLOSORB granules maintain jagged morphology and are surrounded by clot material while (H) ARISTA AH particles show both spherical (dry) morphology and irregularly melded (wet) morphology with a noticeable absence of clot material. (F) Clot material near FLOSORB Granules contains fibrin and red blood cells while (I) clot material entrapped in melded ARISTA AH lacks visible fibrin fibers and is poor in red blood cells. Scale bars: D, G are 1mm; E, H are 200µm; and F, I are 20 µm.
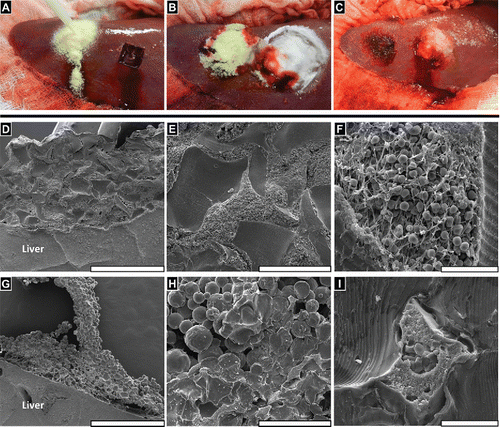
FIGURE 5 FLOSORB (BGP; white bars) provided superior hemostatic success over 10 minutes relative to ARISTA AH (MPH; black bars) and sustained hemostasis over 10 minutes. Superiority was determined by logistic regression with an odds ratio of for hemostatic success of FLOSORB relative to ARISTA AH was 15.18 (95% CI: 7.37 to 31.27). The 95% lower limit of the odds ratio was greater than 1. This indicates that FLOSORB is superior to ARISTA AH (p < 0.001).
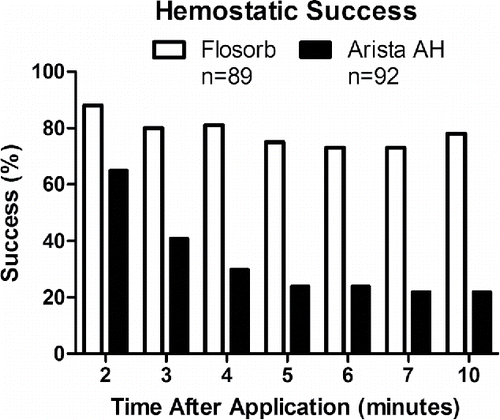
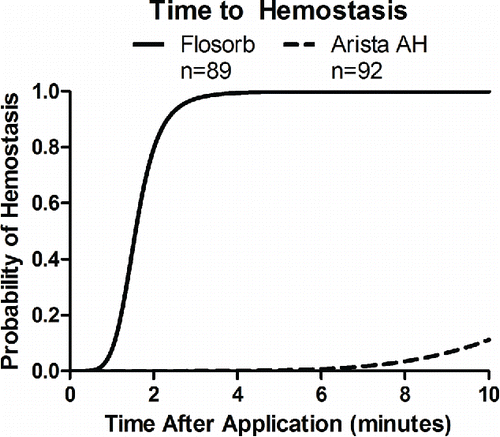
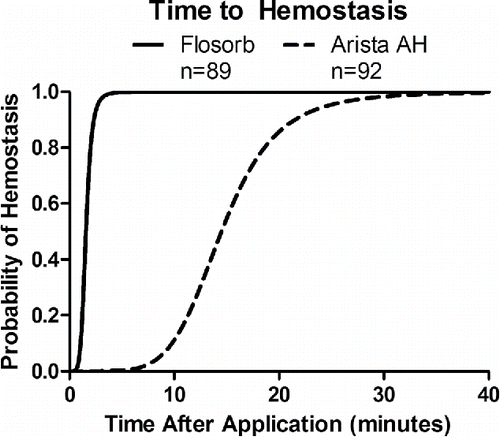
FIGURE 6 Statistical model-estimated probability of hemostasis over time with FLOSORB (BGP; solid line) compared to ARISTA AH (MPH; dashed line) controlled for pre-treatment bleeding level (mild to moderate). Time to hemostasis was 9.23 times faster (95% CI: 6.99 to 12.19) with FLOSORB. The median time to hemostasis (50%) for FLOSORB was inferred from this model to be 1.57 minutes with hemostasis likely to be complete (99%) at 3.65 minutes. The median time to hemostasis (50%) for ARISTA AH was inferred form this model to be 14.5 minutes with hemostasis likely to be complete (99%) at 33.7 minutes (Supplemental Figure S2).