Figures & data
Figure 1. FOXO3a is differentially expressed in cervical cancer tissues and cells. (A) The TCGA database for predicting FOXO3a expression in cervical cancer tissues, and FOXO3a expression was high in cervical cancer tissues. (B) qRT-PCR test for verifying FOXO3a expression in cervical cancer tissues and adjacent normal tissues, and there was increased FOXO3a expression in cervical cancer tissues in comparison to adjacent normal tissues. (C and D) qRT-PCR and western blot experiments for testing FOXO3a expression in human cervical epithelial immortalized cell line H8 and human cervical cancer cell lines (Caski, HeLa, SiHa, C33A, and ME-180), and FOXO3a expression was downregulated in the aforesaid cervical cancer cells in comparison to H8 cells. *p < 0.05; **p < 0.01.
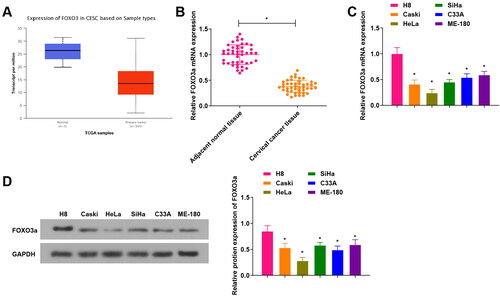
Figure 2. Overexpression of FOXO3a impacts the cervical cancer cell biological functions. (A) FOXO3a transfection efficiency in cells was measured by qRT-PCR, and FOXO3a expression was elevated in cells transfected with oe-FOXO3a in contrast to those transfected with oe-NC. (B) CCK-8 assay for evaluating the cell proliferation capacity upon transfection of oe-FOXO3a and oe-NC. (C) The cell apoptosis rate upon transfection of oe-FOXO3a and oe-NC was measured by flow cytometry. (D and E) The cell migratory and invasive capabilities upon transfection of oe-FOXO3a and oe-NC were assessed by Transwell assay. *p < 0.05.
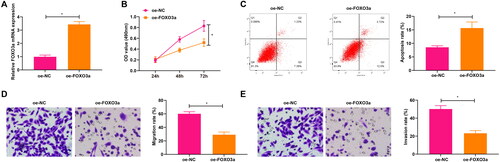
Figure 3. Foxo3a blocks its methylation with the PTEN promoter by inhibiting DNMT3B activity. (A) The binding of Foxo3a to DNMT3B was verified by ChIP test, and sh-FOXO3a treatment had a reduced enrichment of DNMT3B than sh-NC treatment, while oe-FOXO3a treatment had an elevated enrichment of DNMT3B than oe-NC treatment. (B) The methylation level of cells in each group was evaluated by MS-PCR experiment, and there were higher PTEN promoter methylation levels in cervical cancer cells with oe-DNMT3B treatment in comparison to those cells with oe-NC treatment. (C) PTEN mRNA expression after DNMT3B overexpression was tested by qRT-PCR, and decreased PTEN mRNA expression was observed upon oe-DNMT3B treatment in contrast to oe-NC treatment. (D) ChIP assay for examining DNMT3B enrichment in the PTEN promoter region after overexpression and silencing of Foxo3a, and DNMT3B enrichment in the PTEN promoter region was reduced after overexpression of Foxo3a, while there exhibited elevated DNMT3B enrichment in the PTEN promoter region after Foxo3a interference. E. DNMT3B and PTEN expression levels were assessed by qRT-PCR, and there were a decreased DNMT3B expression and an increased PTEN expression after Foxo3a overexpression, while there were an increased DNMT3B expression and a reduced PTEN expression after Foxo3a reduction. *p < 0.05.
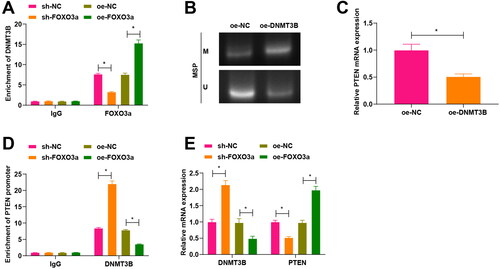
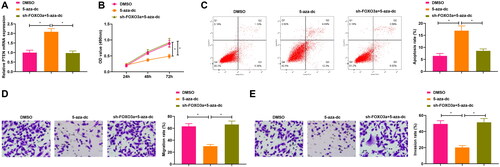
Figure 4. Suppression of PTEN methylation impacts the cervical cancer cell biological functions. A. PTEN expression in cells upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone was measured by qRT-PCR. B. CCK-8 assay for evaluating the cell proliferation capacity upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. C. The cell apoptosis rate was measured by flow cytometry upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. D-E. The cell migratory and invasive capabilities were assessed by Transwell assay upon sh-Foxo3a and 5-aza-dc treatment, and 5-aza-dc treatment alone. *p < 0.05.