Figures & data
Table 1. Oxide chemistry of several synthetic vitreous fibers (SVF) used in this study.
Table 2. Solution chemistry of the simulant fluids used within this work.
Figure 1. Schematic of the updated optical method cell. The fibers were mounted across the slot located mid-cell. Design schematics are provided in the Supplemental Materials.

Figure 2. Typical overview of the flow cell with several fibers mounted in the fluid path (SVF: QFHA22, Fluid: PSF) with the arrows annotating single fibers.
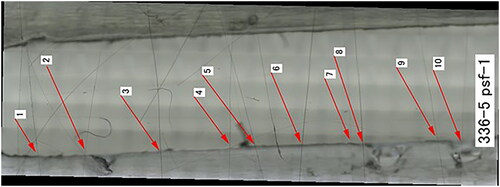
Figure 3. Typical micrographs of an individual fiber during testing. The red box represents the length over which the diameter was measured at each time step.

Table 3. Dissolution rate constant data from the screening experiment of QFHA19 fiber, showing significant increase in kdis with citrate addition.
Table 4. Comparing measured dissolution rate constants obtained for several mineral fibers to those calculated from the Eastes model from in vivo data.
Figure 4. The dissolution rate constant, kdis, calculated by EquationEquation (3)(3)
(3) using the EU 4.5 coefficients compared to the measured value. The solid line is the ideal fit; the 80% confidence interval is an interval of x/3.9.
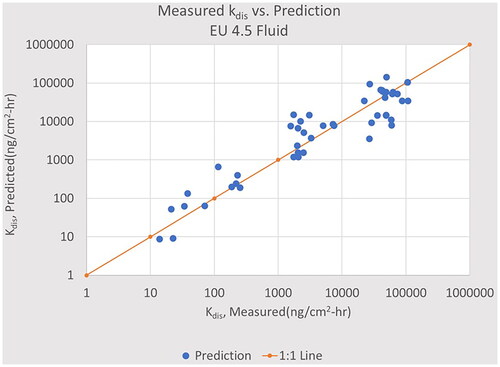
Figure 5. The dissolution rate constant, kdis, calculated by EquationEquation (3)(3)
(3) using the PSF coefficients compared to the measured value. The solid line is the ideal fit; the 80% confidence interval is an interval of x/6.9. PSF, Stefaniak’s Phagoloysosmal Simulant Fluid.
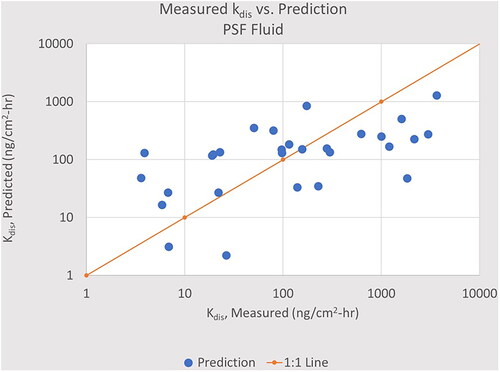
Table 5. Coefficients for the calculation of the fiber dissolution rate constant, kdis, in EU 4.5 and PSF.
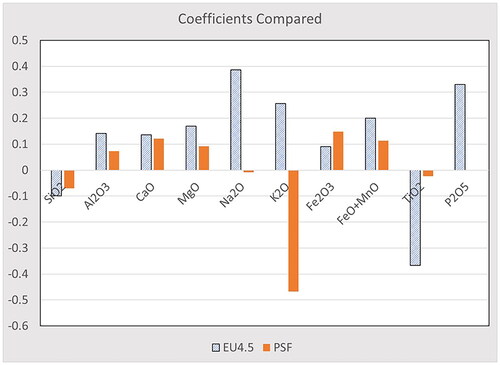
Figure 6. Visual comparison of the coefficients by fluid, showing general agreement within the major oxides.
Supplemental Material
Download MS Excel (16.8 KB)Supplemental Material
Download MS Excel (48 KB)Supplemental Material
Download JPEG Image (88.8 KB)Data availability statement
All data generated or analyzed during this study are included in this published article [and its Supplementary Information Files].
