Figures & data
Figure 1. Representative exposures. The graph (A) represents the variation of PM2.5 for the 5-day WS exposures. The target levels were either 5 mg/m3 (gray circles) or 1 mg/m3 (black squares) where the average levels over 5 days were 5.05 mg/m3 and 1.01 mg/m3 (±sem), respectively. The exposures for these studies utilized the 5 mg/m3concentration. Additionally, the table (B) shows the size distribution of the PM particles and illustrates the rationale for using PM2.5 as the benchmark for characterizing the exposures based on this concentration where the majority (>99%) of the particles are 2.5 µm or smaller.
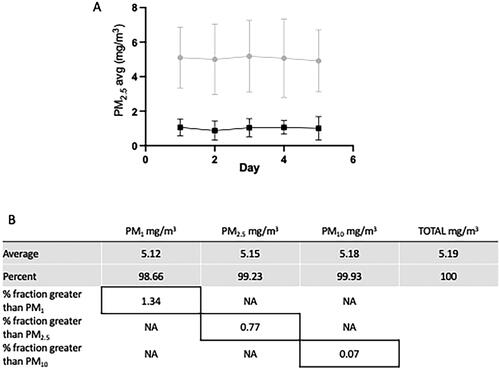
Figure 2. Sex differences in lung function following WS exposures. C57Bl/6 mice were exposed to WS (inhaled) 2 hrs per day for 5 days at a concentration of 5 mg/m3 (PM2.5) or filtered ambient air (FAA). Animal lung functions were then assessed with a BUXCO system by the above listed parameters: resistance and dynamic compliance. Lung function assessments were performed at 24 hrs and 2 months post exposure. As illustrated in the above figure, the lung function was unaffected in the female mice, but significantly (**p < 0.01; ****p < 0.0001) altered in the male mice at the later timepoint (n = 6-12 ± sem).
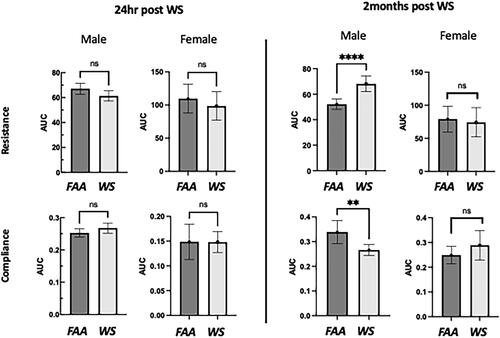
Figure 3. Sex differences in IL-33 expression. Following exposures to WS or FAA, mice were lavaged and assayed for expression of IL-33 in the supernatant by ELISA. There was a WS-associated increase in IL-33 in both male and female lavage fluid and was significantly increased (*p < 0.05)in females. In addition, there was an observed sex effect with higher levels in females with both control (FAA) and WS-exposed groups (††††p < 0.0001, †p < 0.05). There was no significant interaction between sex and exposures. These are at 24 hr following WS exposure with no differences in either sex at 2 months (n = 4-8, ±sem).
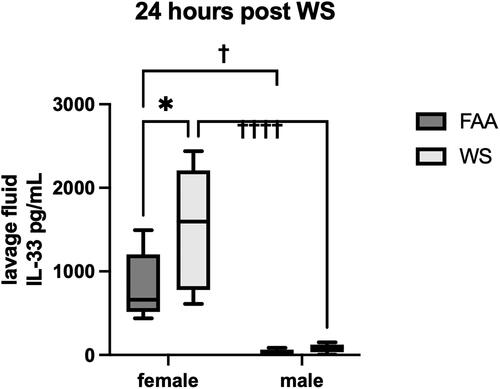
Figure 4. IL-33 effect on male lung function following WS exposures. Male mice were treated with IL-33 as described at the beginning of the WS exposures, and assessed for lung function changes at 24 h and 2 months post. There were no WS-induced changes to lung functions (n = 4-8 ±sem).
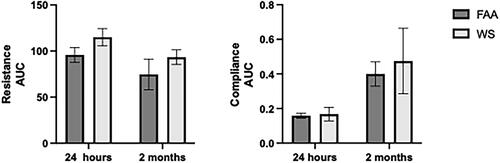
Figure 5. Eosinophilia following IL-33 instillation. Male mice were instilled with IL-33 at days 0 and 1 of the WS exposure. Cellular infiltrates were assessed by cytospin at 24 h and 2 months post WS exposure. There was significant eosinophilia at the 24 h timepoint in both the FAA control mice and the WS-exposed mice as compared with both male and female mice not instilled with IL-33. Additionally, while there was a decrease in the percentage AM in the total lavaged cellular population, the absolute numbers were statistically unaffected by the IL-33 (****p < 0.001; n = 4-8).
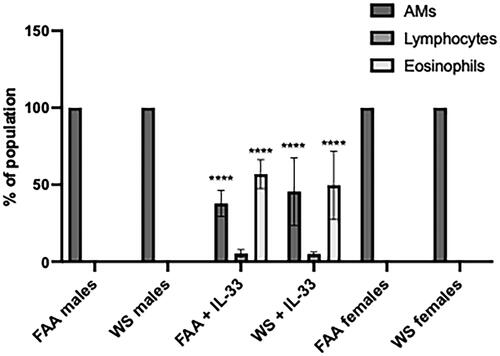
Figure 6. Systemic inflammatory effects. Following WS exposure, male mice were assayed for a panel of inflammatory mediators in serum at 24 h (A) and 2 months (B) post WS exposure. While several showed detectable levels, only KC/GRO levels showed a WS effect in the serum at 24 h post exposure (****p < 0.001; n = 4-8 ± sem).
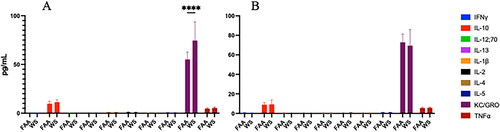
Figure 7. Ex vivo LPS stimulation of WS-exposed AM. Following exposure to WS or FAA, AM were isolated from both male and female mice by whole lung lavage. AM were aliquoted to 96-well plates at 1x105 cells/well and stimulated with LPS (10 ng/mL) for 24 h. Supernatants were assayed for TNFα. as illustrated in the figure, there was a significant (*p < 0.05; ***p < 0.001) increase in TNFα production from male WS-exposed AM at both 24 h and 2 months post exposure (a), but not in the IL-33 males at 2 months (B) or the female AM (C) (n = 4-8 ±sem).
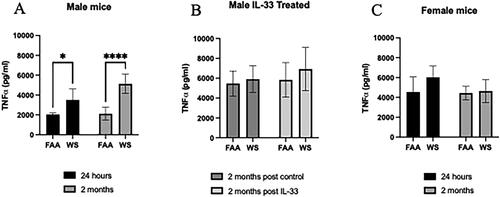
Figure 8. Ex vivo assessment of efferocytosis. Following exposure to WS or FAA, AM were isolated from both male and female mice by whole lung lavage. Fluorescently labeled AM were incubated with fluorescently labeled apoptotic epithelial cells for 4 h. Efferocytosis, phagocytosis of apoptotic cells, was measured by quantifying the percent of double-positive macrophages using flow cytometry. As shown in the figure, the percent of double-positive AM was significantly reduced (*p < 0.05, **p < 0.01) in the male WS-exposed groups at both 24 h and 2 months post exposure. The WS effect was abrogated at the 2-month timepoint by IL-33 treatment (†††p < 0.001). In addition, there was no WS effect on efferocytosis in the female mice (n = 4-6 ±sem).
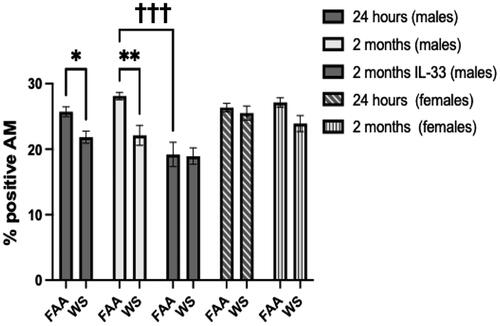
Figure 9. Summary of differentially expressed gene (DEG) numbers. AMs were isolated from mice 24 h or 7 days after exposure to WS or FAA. RNA was isolated and processed for RNA-seq, and DEGs were called by comparing expression in WS-exposed to FAA control using DESeq2 (padj<0.05; Wald test, Benjamini–Hochberg adjusted; n = 3). Total number of WS-responsive genes for each sex/timepoint are represented in stacked bar graph, with number upregulated in yellow and number downregulated in purple.
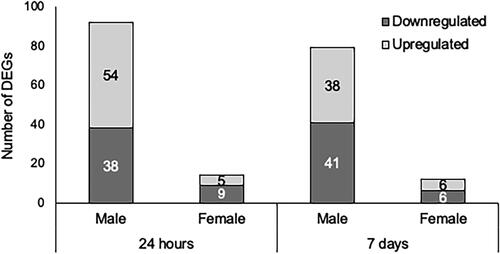
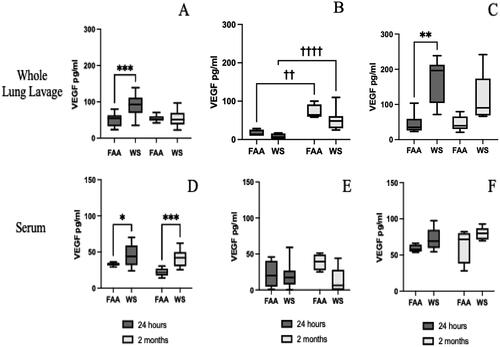
Figure 10. Vascular endothelial growth factor (VEGF) production following WS exposures. Both lavage fluid and serum were assayed by ELISA for VEGF following WS exposure. Samples were collected and frozen until assayed by ELISA. As shown in the graphs, VEGF levels were significantly (***p < 0.001, **p < 0.01) increased in the acute (24 h) phase following WS exposures, in both males and females (A, C respectively), but not in IL-33-treated males (B). However, there was a significant IL-33 effect (†††p < 0.001, ††p < 0.01). Additionally, there was a significant increase in VEGF expression at both the acute (*p < 0.05) and sustained (2 months; ***p < 0.001) timepoints in male mice (D) but not in IL-33 treated male mice (E) or female mice (F) (n = 4-8 ±sem).
Supplemental Material
Download MS Excel (28.8 KB)Data availability
The authors confirm that the data supporting the findings of this study are available within the article and can be shared upon request.
