Figures & data
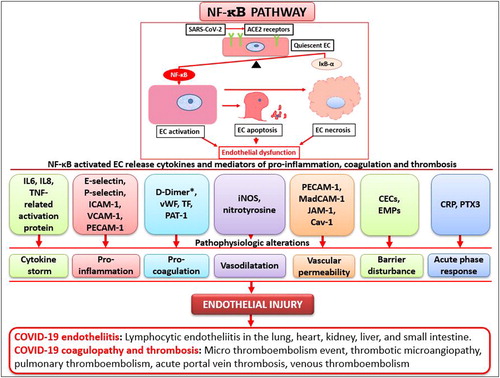
Figure 1. Proposed nuclear factor-κB (NF-κB) pathway by which angiotensin-converting enzyme 2 (ACE2) receptors induce endothelial dysfunction in COVID-19. The balance of NF-kB and its protective gene (IkB-a) maintain endothelial quiescence under normal conditions, but after SARS-Cov-2 binds with ACE2 receptors on the surface of endothelial cells (ECs) and NF-kB activation, the IkB-a genes are insufficient to counteract the action of NF-kB, and ECs become activated. If uncontrolled, ECs progress to apoptosis, necrosis, and endothelial dysfunction. The EC phenotypic changes via the NF-κB pathway result in the release of a variety of molecules/proteins (e.g., IL6, IL8, TNF-related activation protein; E-selectin, P-selectin, ICAM-1, VCAM-1, PECAM-1; D-Dimer, vWF, TF, PAT-1; iNOS, nitrotyrosine; PECAM-1, MadCAM-1, JAM-1, Cav-1; CECs, EMPs; CRP, PTX3). These molecules can directly act on ECs to induce a cytokine storm, inflammation, coagulation, vasodilatation, increased vascular permeability, barrier disturbance, and acute phase response. This endothelial injury can clinically manifest as COVID-19–associated endotheliitis, coagulopathy, and thrombosis. Note that D-dimers are not released from activated ECs and they are one of the fragments produced when plasma cleaves fibrin to break down clots.