Figures & data
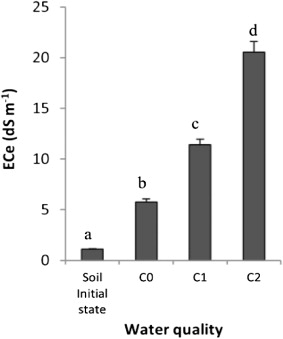
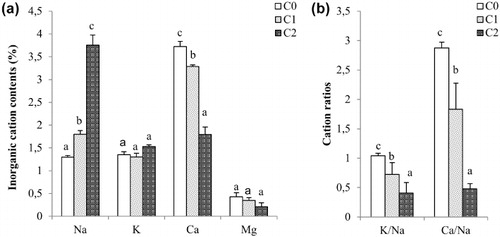
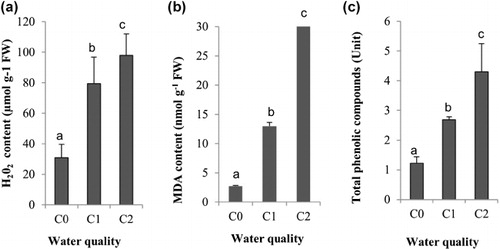
Table 1. The salt effects on the Aloe leaf growth. Irrigations were done with different quality of water: C0 (ECw 1.25 dS m–1), C1 (ECw 3.50 dS m–1) and C2 (ECw 12.00 dS m–1). Values represent means ± standard deviation (SD) of triplicates.