Figures & data
Figure 1. Expression of IL-6 in the stroma cells and a partially intact monolayer of RPE cells in human choroidal fibrovascular tissues from a patient with exudative AMD. Immunohistochemistry shows expression of IL-6 (B) in surgically excised AMD choroidal neovascular tissues, in the stroma (red arrowheads) and a partially intact monolayer of RPE cells (yellow arrows). The specificity of staining is confirmed by the absence of staining with isotype control IgG (A). Scale bars, 50 μm.
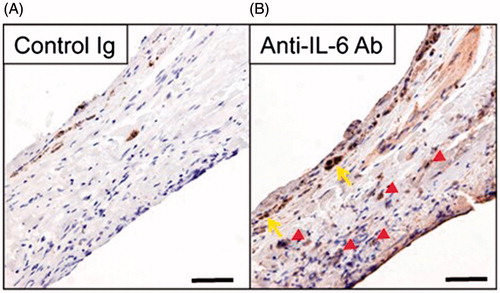
Figure 2. Neutralization of IL-6 R suppressed subretinal fibrosis. The area of subretinal fibrosis was reduced upon the intravitreal injection of neutralizing anti-IL-6 R antibody (MR16-1) compared to isotype control IgG (n = 3). The data are shown as means ± SD. The experiment was repeated three times with similar results. *p < .05.
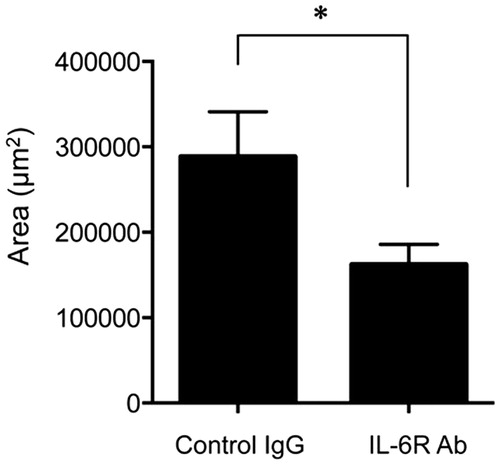
Figure 3. Effects of knockdown of the IL-6 gene on subretinal fibrosis. (A) Expression of the IL-6 gene was assessed in the eyes of WT mice injected with IL-6 siRNA in a dose-dependent manner and were compared with the results for the scramble siRNA-injected eyes in the subretinal fibrosis model (n = 3). (B) The area of subretinal fibrosis was decreased upon intravitreal injection of IL-6-siRNA compared with that observed upon injection of control-siRNA (n = 3). The data are shown as means ± SD. The experiment was repeated three times with similar results. *p < .05.
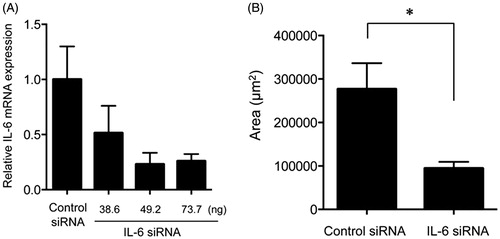
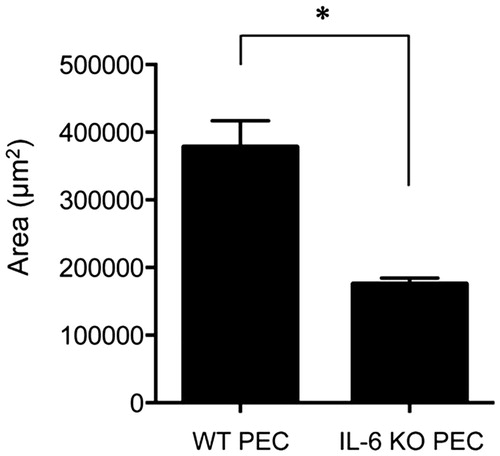
Figure 4. Subretinal fibrosis upon administration of IL-6−∕− PECs. The area of subretinal fibrosis in the eyes of the WT mice was reduced upon the subretinal injection of IL-6−∕− PECs when compared with that observed upon injection of IL-6+/+ PECs (n = 3). The data are shown as means ± SD. The experiment was repeated three times with similar results. *p < .05.