Figures & data
Fig. 1. Formation of a tofu-like precipitate by the addition of various metal chlorides.
Note: Various metal chlorides, magnesium, calcium, copper, ferrous, ferric, and manganese chlorides, were added to soymilk to a final concentration of 20 mM. The mixture was separated into a supernatant and precipitate by centrifugation at 4,100 × g for 10 min at 4 °C. (A) Separation was observed. (B) The precipitation efficiency is expressed as the percentage weight of the wet precipitate to that of the initial soymilk. Data are shown as the average ± standard deviation of five independent experiments.
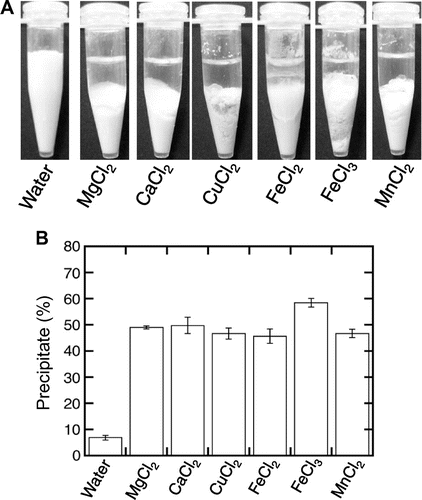
Fig. 2. Metal chloride concentration-dependent changes in the precipitate formation and supernatant protein concentration.
Note: Magnesium chloride (A), calcium chloride (B), copper chloride (C), ferrous chloride (D), ferric chloride (E), and manganese chloride (F) were added to soymilk at various concentrations. The mixture was separated into a supernatant and precipitate as described in the Materials and methods section. The precipitation efficiency (open circles) and supernatant protein concentration (closed circles) were determined as described in the Materials and methods section. Data are shown as the average ± standard deviation of three independent experiments.
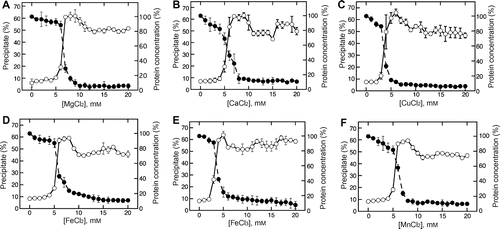
Fig. 3. Aspects of the precipitate resulting from the addition of a metal chloride.
Note: The precipitates were prepared from an equal volume (0.9 mL) of soymilk (A) as already described. Magnesium chloride was added at 9.0 mM (B) and 12.0 mM (C). Calcium chloride was added at 8.0 mM (D) and 13.0 mM (E). Copper chloride was added at 6.0 mM (F) and 12.0 mM (G). Ferrous chloride was added at 7.0 mM (H) and 12.0 mM (I). Ferric chloride was added at 5.0 mM (J) and 8.0 mM (K). Manganese chloride was added at 7.0 mM (L) and 13.0 mM (M). White bars show 1 cm.
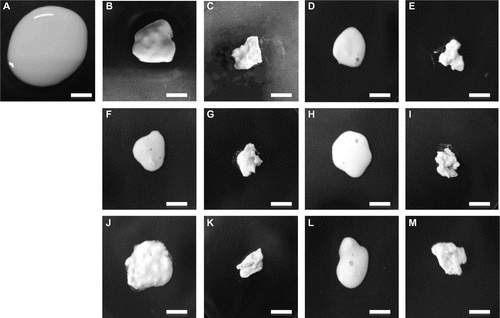
Table 1. Differences in the % precipitate and % RWC between SP and RP.
Table 2. Midpoint concentrations for changes in the WPW and protein concentration, and the stability constants for the EDTA-metal complexes.
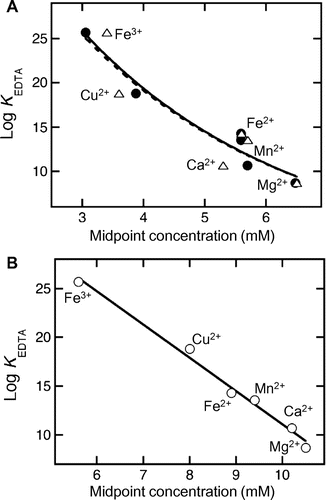
Fig. 4. Correlation between the changes in WPW and protein precipitation with the stability constants of EDTA for the metal ions.
Note: The respective stability constants of EDTA (log KEDTA) for Ca2+, Cu2+, Fe2+, Fe3+, Mg2+, and Mn2+ were 10.70, 18.8, 14.3, 25.7, 8.69, and 13.56.Citation17) A, The Cm value for the transition of WPW (closed circles) and protein concentration reduction (open triangles) with SP formation were calculated from Fig. by a sigmoidal function, using KaleidaGraph 3.6 software (Synergy Software, PA, USA). Plots for the precipitate efficiency (solid line) and protein concentration reduction (dashed line) were fitted by an exponential function with the same software. B, The Cm value for the decrease in WPW with RP formation (open circles) was calculated from Fig. by a sigmoidal function with, KaleidaGraph 3.6 software. The plots were fitted by a linear function with the same software (solid line).