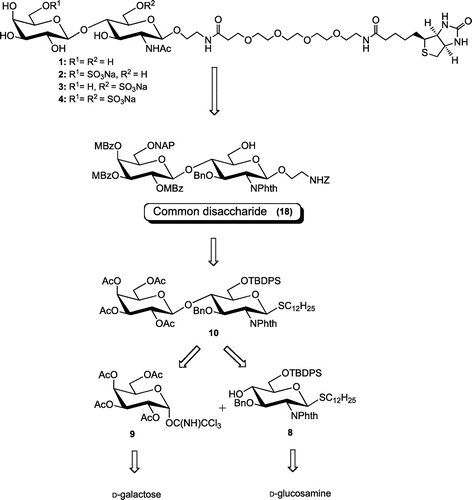
Figures & data
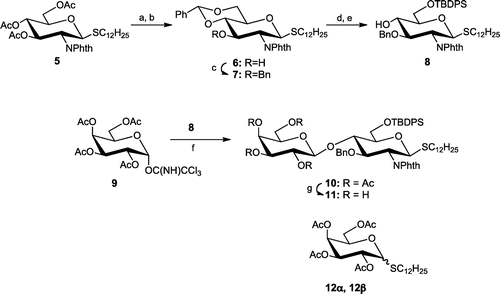
Scheme 2. Synthesis of disaccharide 11.
Note: Reagents and conditions: (a) NaOMe, MeOH; (b) PhCH(OMe)2, p-TsOH, 78% (2 steps); (c) BnBr, NaH, DMF, 0 °C-rt, 86%; (d) camphorsulfonic acid, CH2Cl2-MeOH; (e) TBDPSCl, imidazole, DMF, 69% (2 steps); (f) TMSOTf, CH2Cl2, −20 or −78 °C; (g) NaOMe, MeOH, 60% (2 steps).
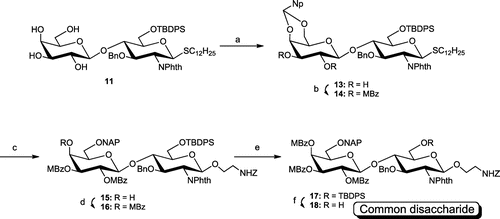
Scheme 3. Synthesis of common disaccharide 18.
Note: Reagents and conditions: (a) 2-naphthaldehyde, p-TsOH·H2O, CH3CN, 89%; (b) MBzCl, DMAP, pyridine-CH2Cl2, quant.; (c) BH3·Me3 N, AlCl3, cat. H2O, THF, 92%; (d) MBzCl, DMAP, pyridine-CH2Cl2, 98%; (e) NIS, TfOH, MSAW300, Et2O-(CH2Cl)2, quant.; (f) 1 M tetra-n-butyl ammonium fluoride, AcOH, THF, 79%.
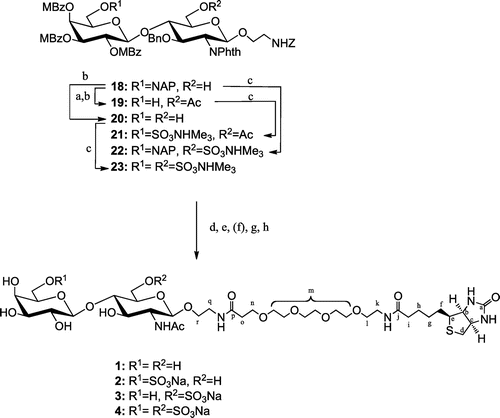
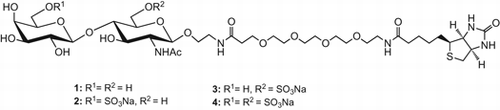
Scheme 4. Synthesis of target compounds 1–4.
Note: Reagents and conditions: (a) Ac2O, pyridine, quant.; (b) DDQ, cat. H2O, CH2Cl2-MeOH, 84% for 19 (2 steps), 78% for 20; (c) SO3· NMe3, DMF, 60 °C, 88% for 21, quant. for 22, 90% for 23; (d) 1,3-diaminopropane, EtOH, reflux; (e) Ac2O, Et3N, MeOH; (f) NaOH, MeOH (for 21, 22), 50% MeOH (for 23), reflux; (g) H2, Pd-black, aq. EtOH + dil. AcOH (for 18), aq. 2-PrOH + dil. AcOH (for 21, 22), dil. AcOH (for 23); (h) NHS-PEG4-biotin, 1 M Na3PO4, 0.15 M NaCl.