Figures & data

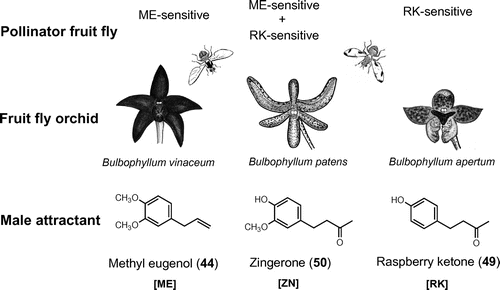
Fig. 1. Plant metabolites as host-finding cues in butterflies and aphids.
Note: Oviposition stimulants of P. xuthus: hesperidin (1), narirutin (2), rutin (3), vicenin-2 (4), adenosine (5), 5-hydroxy-Nω-methyltryptamine (6), bufotenine (7), (−)-synephrine (8), (+)-chiro-inositol (9), (−)-stachydrine (10); P. macilentus: cnidioside A (11); P. bianor: (−)-2C-methyl-D-erythrono-1,4-lactone (12), (−)-4-(E)-caffeoyl-L-threonic acid (13); P. polyxenes: luteolin 7-O-(6″-O-malonyl)-β-D-glucopyranoside (14), tyramine (15), chlorogenic acid (16); A. alcinous: aristolochic acid I (17), sequoyitol (18), 3-hydroxy-4-methoxycinnamoylmalic acid (19); B. philenor: 17 and pinitol (20); L. japonica: isorhamnetin 3-O-glucopyranosyl-(l→6)-galactopyranoside-7-O-glucopyranoside (21). Larval-feeding stimulant of P. xuthus: citrusin I (22), isosinensetin (23), 1,2-dilinolenoyl-3-galactopyranosyl-sn-glycerol (24). Probing stimulants in an aphid, M. crassicauda: quercetin 3-O-α-L-arabinopyranosyl-(1→6)-(2″-O-(E)-p-coumaroyl)-β-D-galactopyranoside (25).
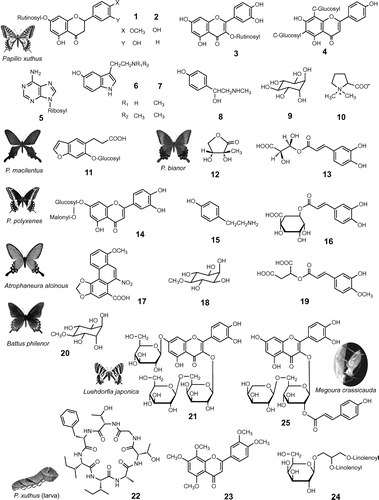
Fig. 2. Plant metabolites as chemical barrier against insects.
Note: Oviposition and feeding deterrents of P. xuthus: quercetin 3-O-(2G-β-D-xylopyranosylrutinoside) (26), 5-{[2-O-(β-D-apiofuranosyl)-β-D-glucopyranosyl]oxy}-2-hydroxybenzoic acid (27) and disyringoyl glucaric acid (28). Probing deterrent in aphids, M. crassicauda: (E)-2-methyl-2-butene-1,4-diol 4-O-β-D-glucopyranoside (29). Insect growth regulators: juvenile hormone III (30), juvocimene II (31), 1-(3,4-methylenedioxyphenyl)-(E)-3-decene (juvadecene) (32).
![Fig. 2. Plant metabolites as chemical barrier against insects.Note: Oviposition and feeding deterrents of P. xuthus: quercetin 3-O-(2G-β-D-xylopyranosylrutinoside) (26), 5-{[2-O-(β-D-apiofuranosyl)-β-D-glucopyranosyl]oxy}-2-hydroxybenzoic acid (27) and disyringoyl glucaric acid (28). Probing deterrent in aphids, M. crassicauda: (E)-2-methyl-2-butene-1,4-diol 4-O-β-D-glucopyranoside (29). Insect growth regulators: juvenile hormone III (30), juvocimene II (31), 1-(3,4-methylenedioxyphenyl)-(E)-3-decene (juvadecene) (32).](/cms/asset/bc2ede77-b3d8-4597-a882-b3034d6179b6/tbbb_a_877836_f0002_b.gif)
Fig. 3. Defensive substances sequestered from plants.
Note: I. leuconoe: ideamine B (33); P. apollo and A. glossulariata: sarmentosin (34); A. gaschkevitchii arichannatoxin I (35); A. nipponicus: paederoside (36); A. rosae ruficornis: clerodendrin D (37); D. speciosa and C. arcuata: 23,24-dihydrocucurbitacin D (38).
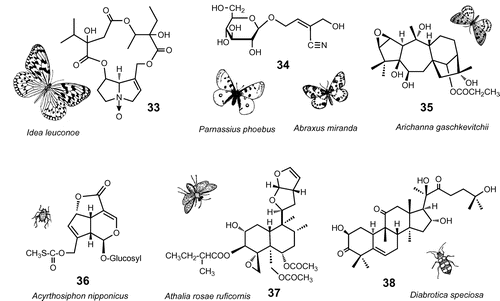
Fig. 4. Plant chemicals for sexual communication.
Note: Males of the giant danaine butterfly, I. leuconoe, biotransform defensive pyrrolizidine alkaloid, lycopsamine (39), to danaidone (40) and viridifloric β-1actone (41) and emit from the hairpencil organ as sex pheromone. A female oriental fruit moth, G. molesta, is attracted to male sex pheromone composed of methyl epijasmonate (42) and ethyl (E)-cinnamate (43). Males of oriental fruit fly, B. dorsalis, pharmacophagously acquire methyl eugenol (44) from plants and biotransform to sex pheromone 2-allyl-4,5-dimethoxyphenol (45) and (E)-coniferyl alcohol (46), which entice females during courtship. Males of the guava fruit fly, B. correcta, sequester β-caryophyllene (47) in the rectal pheromone glands. Mediterranean fruit fly, C. capitata, may use α-copaene (48) as a cue to navigate both sexes to the rendezvous site.
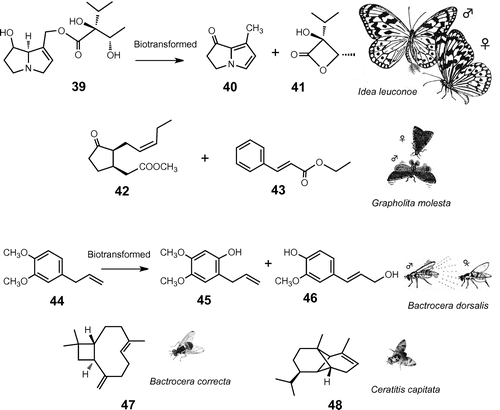
Fig. 5. Floral synomone of fruit fly orchids attracting pollinator Bactrocera fruit flies.
Note: B. vinaceum emits methyl eugenol (ME, 44); B. apertum produces raspberry ketone (RK, 49); B. patens emits zingerone (ZN, 50) which has a hybrid structure between 44 and 49 and attracts both ME-sensitive (e.g. oriental fruit fly, B. dorsalis) and RK-sensitive species (e.g. melon fly, B. cucurbitae).