Figures & data
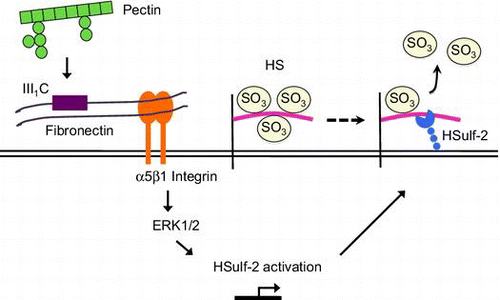
Fig. 1. Alteration of disaccharide composition of HS at the surface of differentiated Caco-2 cells after pectin administration.
Notes: The disaccharide composition of HS on the cell surface of differentiated Caco-2 cells was compared between the cells with and without pectin treatment. The disaccharide mixtures of HS were analyzed by HPLC with a UV detection system. The values are shown as means ± SD of three independent experiments. After the percentage data were converted to arcsin values, statistical analyses were performed by Tukey’s test. *p < 0.05, **p < 0.01.
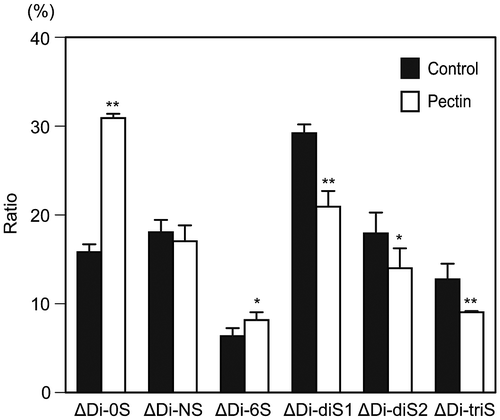
Fig. 2. Effects of pectin on the mRNA expression of HSulf-1 and HSulf-2 in the differentiated Caco-2 cells.
Notes: (A) Differentiated Caco-2 cells were incubated with 0.1 mg/mL pectin for 1 h, and then incubated with normal medium for 3, 9, 18, and 24 h. Total RNA was collected from the differentiated Caco-2 cells and the mRNA expression levels of HSulf-1 and HSulf-2 were analyzed by RT-PCR. The mRNA expression of the cells without pectin administration was shown as 0 h. (B) HSulf-2 mRNA expression of differentiated Caco-2 cells was quantitatively determined by real-time PCR and normalized relative to GAPDH expression. The values are shown as means ± SD of at least three independent experiments. Statistical analyses were performed by Tukey’s test. **p < 0.01.
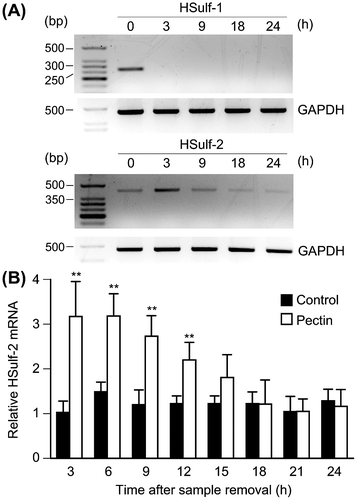
Fig. 3. Effects of pectin on 6-OSTs and 2-OST-1 mRNA expression level in the differentiated Caco-2 Cells.
Notes: Differentiated Caco-2 cells were incubated with 0.1 mg/mL pectin for 1 h, and then incubated with normal medium for the indicated times. The time courses of changes in 6-OST-1, 6-OST-2, and 2-OST-1 mRNA expression were quantitatively determined by real-time RT-PCR and normalized relative to GAPDH expression. The values are shown as means ± SD of at least three independent experiments. Each value was compared with each control and statistical analyses were performed by Tukey’s test. *p < 0.05, **p < 0.01.
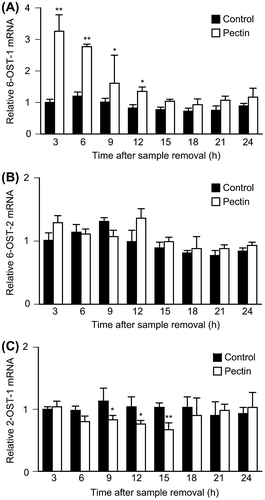
Fig. 4. Effects of FNIII1C and RGD peptides on HSulf-2 mRNA expression level in the differentiated Caco-2 cells.
Notes: (A) Differentiated Caco-2 cells were stimulated by 0.1 mg/mL pectin with or without FNIII1C peptide for 1 h. After the medium was exchanged, cells were incubated for 6 h. Relative mRNA expression of HSulf-2 was measured by real-time RT-PCR. (B) Differentiated Caco-2 cells were stimulated by 0.1 mg/mL pectin with or without RGD peptide for 1 h. After the medium was exchanged, cells were incubated for 6 h. Relative mRNA expression of HSulf-2 was measured by real time RT-PCR. The values are shown as means ± SD of three independent experiments. Statistical analyses were performed by Tukey’s test. **p < 0.01. (C) The expression of α5 integrin in differentiated Caco-2 cells was detected by Western blotting analysis.
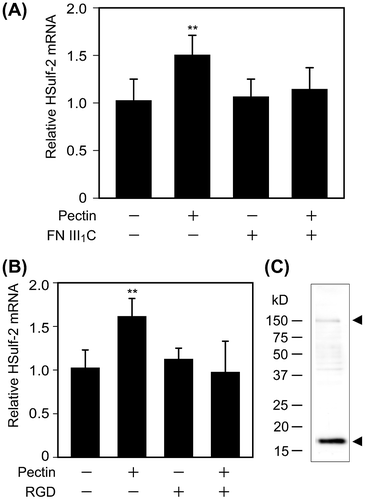
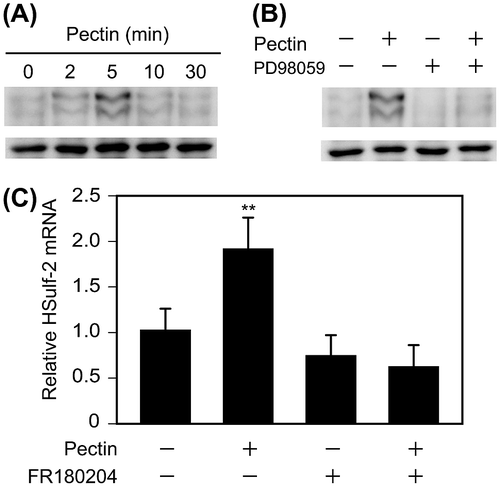
Fig. 5. Effects of pectin on the activation of ERK1/2 and ERK1/2 inhibitor on HSulf-2 mRNA expression level in the differentiated Caco-2 cells.
Notes: (A) Differentiated Caco-2 cells were incubated with 0.1 mg/mL pectin for 0, 2, 5, 10, or 30 min. The time course of changes in pectin-induced phosphorylation levels of ERK1/2 were analyzed by Phos-tag Western blotting analysis. (B) Differentiated Caco-2 cells were preincubated with 50 μm PD98059 and then incubated with 0.1 mg/mL pectin for 0 or 5 min. Phosphorylation levels of ERK1/2 were analyzed by Phos-tag Western blotting analysis. (C) Differentiated Caco-2 cells were pretreated with FR180204 for 2 h followed by incubation with 0.1 mg/mL pectin for 1 h. After the medium was exchanged, the cells were incubated for 6 h. Relative mRNA levels of HSulf-2 were measured. The values are shown as means ± SD of three independent experiments. Statistical analyses were performed by Tukey’s test. **p < 0.01.