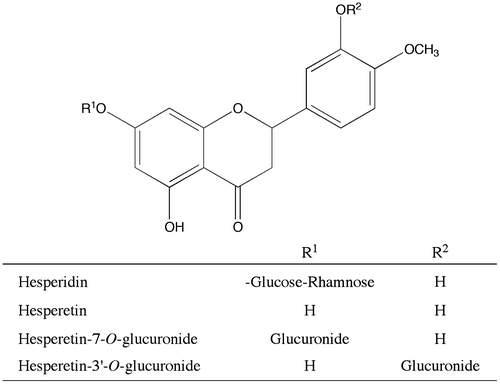
Figures & data

Fig. 2. PPARγ agonist activity of hesperidin, hesperetin, and hesperetin glucuronides.
Notes: COS-1 cells were co-transfected with the luciferase reporter plasmid, p17m2G, which contained a GAL4 upstream-activating sequence in the promoter region, an expression vector for the human PPARγ LBD fused to the GAL4 DNA-binding domain, pPPARγ-GAL4, and SEAP control vector, pSEAP. The results are relative to the luciferase expression level and were normalized to the SEAP activity. Hesperidin derivatives and troglitazone, a positive control PPARγ agonist, were dissolved in DMSO and added to the medium of the transfected cells. Each value is presented as the mean ± standard error of three experiments. *p < 0.05, significantly different from cells adding DMSO.
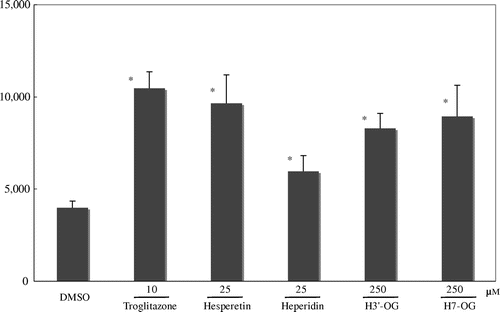
Fig. 3. Adipocyte differentiation accelerative activity of hesperetin glucuronides on 3T3-L1 cells.
Notes: To differentiate 3T3-L1 cells into adipocytes, 4.2 × 104 cells were seeded into each well of a 24-well plate. After 2 d, the cells were treated with 0.5 mM isobutylmethylxanthine, 1 μM dexamethazone, and 1.7 or 2.8 × 10−3 μM insulin for 48 h. The medium was then changed to a fresh medium containing 1.7 or 2.8 × 10−3 μM insulin, with or without hesperidin glucuronides, and was changed thrice every 72 h. Oil Red O staining was used to examine lipid accumulation in the 3T3-L1 cells differentiated into adipocytes. In brief, the cells were fixed with 10% formaldehyde after being washed with phosphate-buffered saline and stained with a saturated solution of Oil Red O in 60% isopropanol solution for 15 min. After removal of the staining solution, the cultures were observed under a microscope (A). (a), (b), (c), and (d) show, respectively, the cells with additions of 2.8 × 10−3 μm insulin, 1.7 μm insulin, 1.7 nm insulin with 10 μm H3′-OG, and 1.7 nm insulin with 10 μm H7-OG. Spectrophotometric quantification of the stain was performed by extracting the stained oil droplets in the cell monolayers with isopropanol. Then, the absorbance was measured at 570 nm (B). This experiment was performed thrice and same results were acquired.
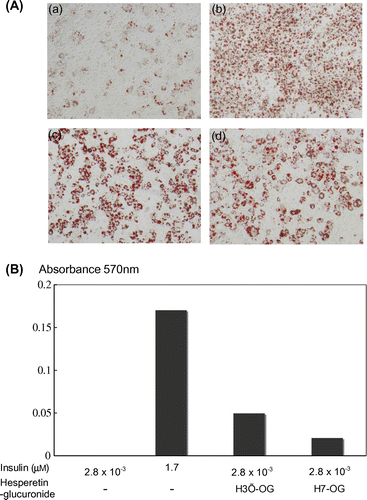
Fig. 4. Upregulation of mRNA expression of adiponectin, C/EBPα, and PPARγ genes in 3T3-L1 cells by hesperetin glucuronides.
Notes: 3T3-L1 cells were differentiated by the addition of 1.7 or 2.8 × 10−3 μm insulin containing 10 μm hesperetin glucuronides. After total RNA was prepared, the adiponectin, C/EBPα, and PPARγ genes were amplified after cDNA synthesis, and the expression levels were observed under a transilluminator after agarose gel electrophoresis and staining with ethidium bromide. The data were photographed using digital camera, and quantitative analysis was performed using Image J ver. 1.47 software (National Institutes of Health, USA).
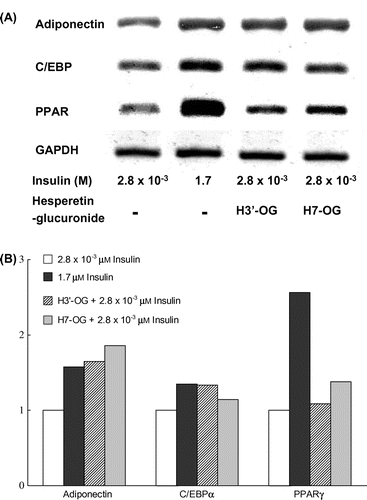
Fig. 5. Additive effect of hesperetin glucuronides with troglitazone on PPARγ agonist activity.
Notes: COS-1 cells transfected with pPPARγ-GAL4 and p17m2G were used to assay each compound at the indicated concentration. Each value represents the mean ± standard error of three experiments.
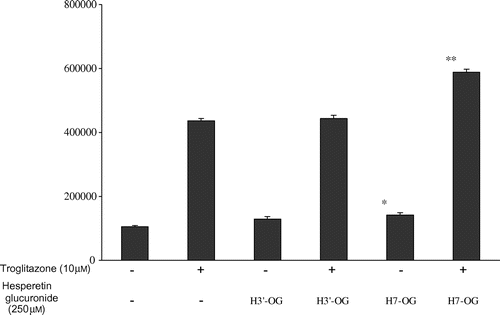
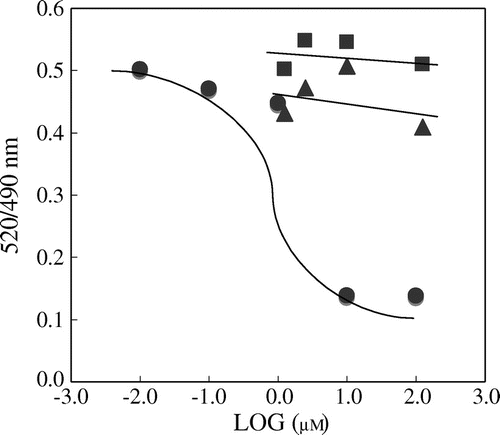
Fig. 6. Hesperetin glucuronides show no competitive activity with a thiazolidinedione derivative against PPARγ
Notes: The LanthaScreen TR-FRET PPARγ assay was performed according to the manufacturer’s instructions. The data for pioglitazone, H7-OG, and H3′-OG are indicated by closed circles, closed triangles, and closed squares, respectively.