Figures & data
Fig. 2. 13C-NMR spectra of model chromophore.
Notes: 13C-labeled DCL reacted with DTT to form the model chromophore. The 13C-labeled sp2 carbon was observed at 135.3 ppm. The signal shifted to 45.4 and 45.2 ppm at the sp3 carbon when the chromophore was formed. Due to the chiral centers of DTT, the chromophore was observed as a mixture of diastereomers. The spectra were recorded in CD3OD.
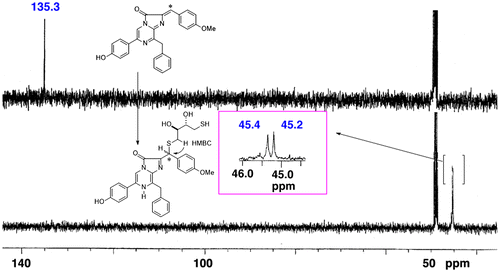
Fig. 3. Mass spectra of the peptides of the chromophore of symplectin.
Notes: The CGLK peptide bound to F-DCL formed the chromophore of symplectin. The signal was observed at m/z 422.78 (M + 2H)2+. The T43 peptide was produced by proteolytic digestion of symplectin with trypsin. The oxidized chromophore was observed at m/z 416.77 (M + 2H)2+ by losing carbon dioxide.
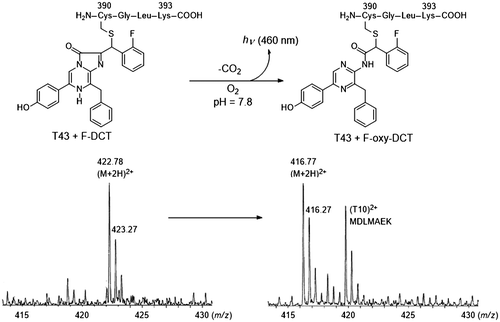
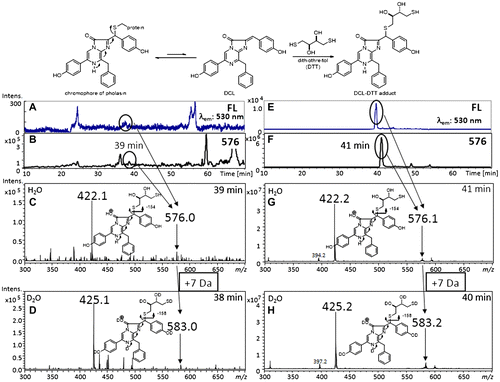
Fig. 4. Preparation of DCL–DTT from the chromophore of pholasin and the LC–MS spectra of extracts obtained from pholasin after being treated with DTT.
Notes: There is an equilibrium between the chromophore of pholasin and DCL. The chromophore was converted to the DCL–DTT adduct by the addition of an excess amount of DTT. The DCL–DTT adduct was extracted from a pholasin solution (upper scheme). (A)–(D) LC–MS spectra of extracts obtained from pholasin and (E)–(H) from the authentic DCL–DTT adduct. (A), (E) Chromatogram monitored by fluorescence (530 nm emission). (B), (F) Ion-chromatogram with m/z 576, which corresponded to the molecular weight of the DCL–DTT adduct. (C), (G) Mass spectra of the ion peak at around 40 min. (D), (H) Mass spectra of the same ion peak (around 40 min) when acetonitrile/D2O was used as the mobile phase for the HPLC.