Figures & data
Fig. 1. Expression, Purification, and Lectin Blot Analysis of His-hDectin-1 Fusion Proteins.
Note: (A) Schematic representation of structural domains of human dectin-1 and His-hDectin-1 fusion proteins. The domain organization is indicated by boxes, as follows: cytoplasmic domain (Cyto), transmembrane domain (TM), neck domain (Neck), and C-type lectin-like domain (CTLD).Citation3,6) SS and His represent signal sequence and His6, respectively. (B) The purified His-hDectin-1A-ECD and His-hDectin-1B-ECD were electrophoresed on a 12% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane. The membrane was stained with anti-His6 antibody. Molecular mass markers are shown to the left. (C) SDS-PAGE and blotting were done as described in B. The membrane was stained with lectins, as indicated. Lanes: 1 and 8, Con A; 2 and 9, PHA-E; 3 and 10, PNA; 4 and 11, RCA-I; 5 and 12, SBA; 6 and 13, UEA-I; 7 and 14, WGA.
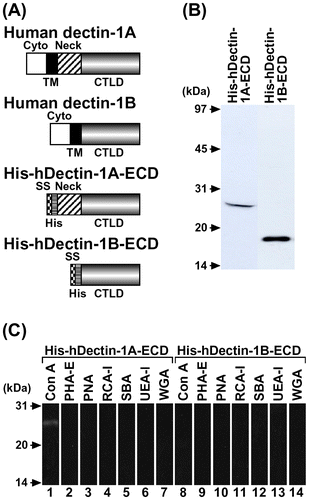
Fig. 2. Carbohydrate Binding Specificity of His-hDectin-1 Fusion Proteins.
Note: The purified His6-tagged human dectin-1 fusion proteins (His-hDectin-1A-ECD and His-hDectin-1B-ECD) were incubated with the indicated insoluble gels. Following incubation, the insoluble gels were pelleted by centrifugation, washed three times, and eluted with Laemmli buffer with DTT. The bound and unbound proteins were analyzed by SDS-PAGE and immunoblotting as described in the legend to Fig. (B).
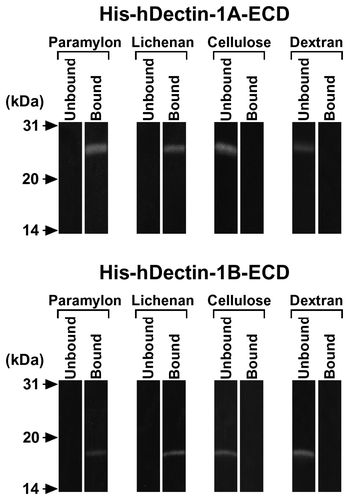
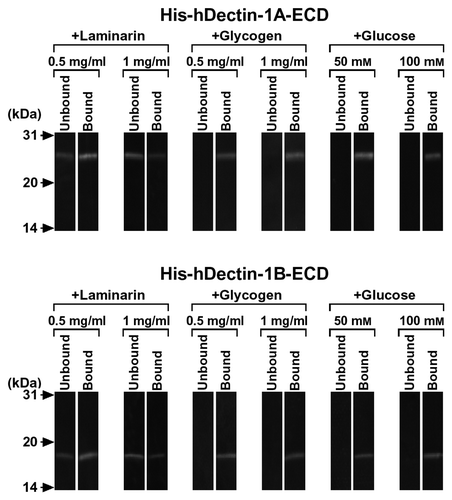
Fig. 3. β-Glucan Binding Specificity of His-hDectin-1 Fusion Proteins.
Note: To determine the binding specificity of human dectin-1, the ability of various carbohydrates to block the β-glucan binding of His-hDectin-1A-ECD or His-hDectin-1B-ECD was examined. Binding assay was done as described in the legend to Fig. 2, except for the addition of competing carbohydrates. Competing polysaccharides (laminarin and glycogen) were present at a concentration of 0.5 or 1 mg/mL in the reaction mixtures, as indicated, to inhibit the binding of His-hDectin-1A-ECD or His-hDectin-1B-ECD to lichenan. Glucose was present at a concentration of 50 or 100 mM in the reactions, as indicated, to inhibit lichenan binding of these recombinant proteins.