Figures & data
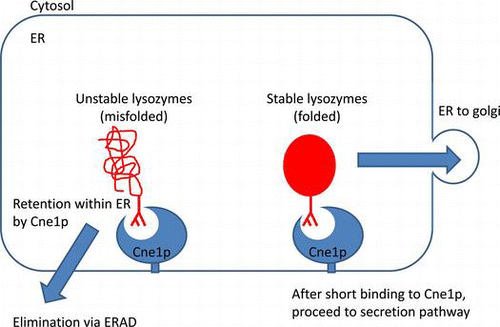
Fig. 1. Restriction map of pUCGPD2 (A) and construction of the expression plasmids for c-Myc-tagged lysozymes (B).
Fig. 3. Secretion of the c-Myc-tagged Mutant Lysozymes G49N and G49N/D66H Expressed in the Wild Type (white column) and CNE1-deficient (black column) S. cerevisiae.
Note: Data are presented as mean ± standard deviation (n = 3).
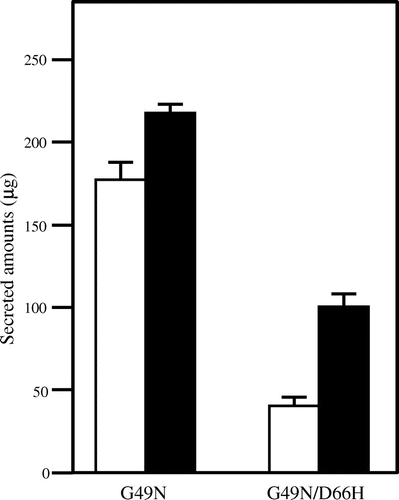
Fig. 4. Immunofluorescence staining of the mutant lysozymes G49N and G49N/D66H expressed in wild type and CNE1-deficient S. cerevisiae using Anti-cMyc epitope antibody.
Notes: (A) Low magnification (×100); (B) high magnification (×1000).
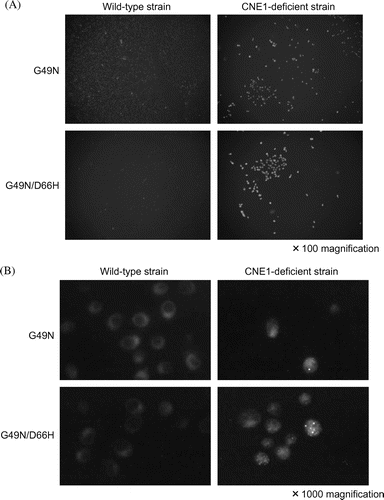
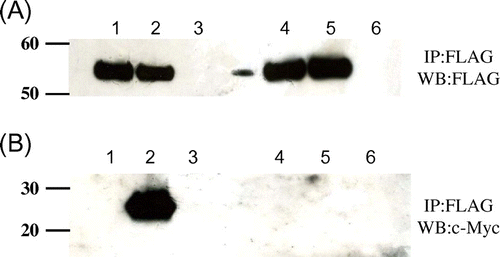
Fig. 5. Co-immunopreciptation of c-Myc-tagged mutant lysozymes and FLAG-tagged soluble Cne1p.
Notes: After immunoprecipitation with anti-FLAG antibody, western blotting was performed using anti-FLAG (A) or anti-c-Myc (B) antibody. Lane 1, soluble Cne1p; lane 2, soluble Cne1p + G49N/D66H lysozyme; lane 3, soluble Cne1p + G49N lysozyme.
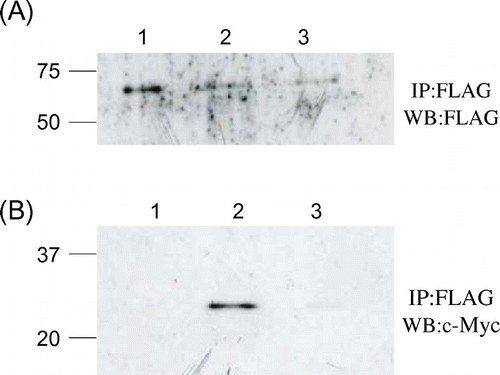
Fig. 6. Co-immunopreciptation of various c-Myc-tagged unstable mutant lysozymes and FLAG-tagged soluble Cne1p.
Notes: After immunoprecipitation with anti-FLAG antibody, western blotting was performed using anti-FLAG (A) or anti-c-Myc antibody (B) antibody. Lane 1, soluble Cne1p + G49N lysozyme; lane 2, soluble Cne1p + G49N/D66H lysozyme; lane 3, soluble Cne1p + G49N/C76A lysozyme; lane 4, soluble Cne1p + K13D/G49N lysozyme.
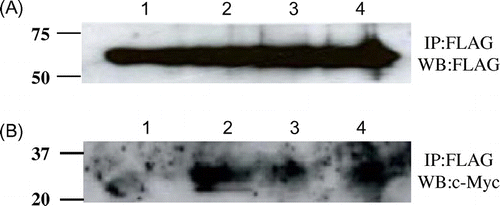
Fig. 7. Co-immunopreciptation of c-Myc-tagged mutant lysozymes and FLAG-tagged soluble Cne1p before and after treatment with tunicamycin.
Notes: After immunoprecipitation with anti-FLAG antibody, western blotting was performed using anti-FLAG (A) or anti-c-Myc (B) antibody. Lanes 1 and 4, soluble Cne1p + G49N lysozyme; lanes 2 and 5, soluble Cne1p + G49N/D66H lysozyme; lanes 3 and 6, G49N/D66H lysozyme. Lanes 1–3, in the absence of tunicamycin; lanes 4–6, in the presence of tunicamycin.