Figures & data
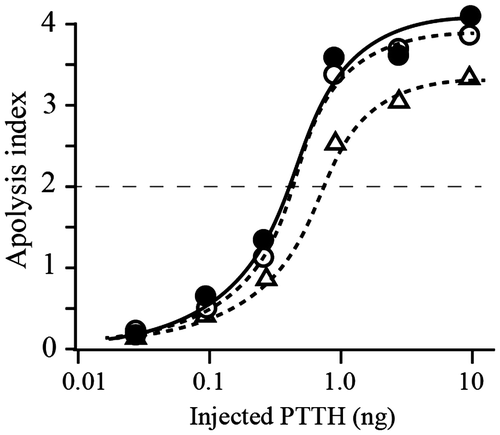
Fig. 1. Analyses of peptides derived from the native and recombinant PTTHs.
Notes: (A) Comparison of the RP-HPLC profiles of lysyl endopeptidase-treated E-rPTTH and B-rPTTH. Peaks A in E-rPTTH and B in B-rPTTH were eluted at different retention times. The peptides corresponding to peaks A and B were further analyzed to determine the structure of the N-glycan. (B) MALDI-TOF MS analyses of the peptides derived from B-rPTTH and E-rPTTH. A possibly N-glycosylated peptide derived from B-rPTTH (a) and E-rPTTH (b) was analyzed. After PNGase F digestion, a peptide corresponding to peak B in Fig. (A) was also analyzed (c). The asterisk indicates an ion peak corresponding to the molecular weight of an N-glycosylated peptide without a deoxyhexose, possibly a fucose residue.
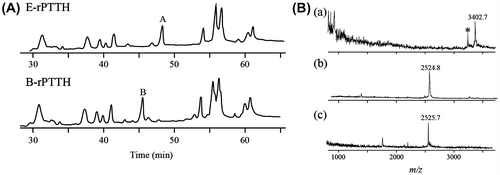
Fig. 2. Structural analyses of PA-N-glycan on PTTHs.
Notes: (A) 2D PA-N-glycan map. The dots indicate the PA-N-glycan derived from B-rPTTH. The arrows indicate the migration on the map after treatment of the PA-N-glycan with α-fucosidase and α-mannosidase. Mapped glucose units were defined by the retention times of the commercially available PA-glucose oligomers. (B) HPLC profiles of PA-N-glycans derived from native PTTH and B-rPTTH using ODS column. Peaks corresponding to PA-N-glycans indicate numbers on peaks. (C) Structures of N-glycan on PTTHs. The percentages of N-glycans are indicated by each fluorescent peak intensity of PA-derivatives observed in (B).
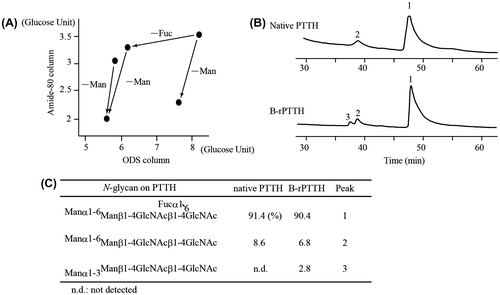
Fig. 3. Biological activities of the native and recombinant PTTHs.
Notes: Dose-dependent activity was observed using three different PTTHs. The biological activity of PTTH was evaluated using the average of the apolysis index (N = 7); points 4, 3, 2, or 1 were observed if apolysis was observed on the 3rd, 4th, 5th, or 6th days after sample injection into the brainless pupae, respectively.Citation6) Closed circles, open circles, and open triangles indicate the effects of native PTTH, B-rPTTH, and E-rPTTH, respectively. The biological activities of these PTTHs were shown to be reproducible using a different preparation of brainless pupae.