Figures & data
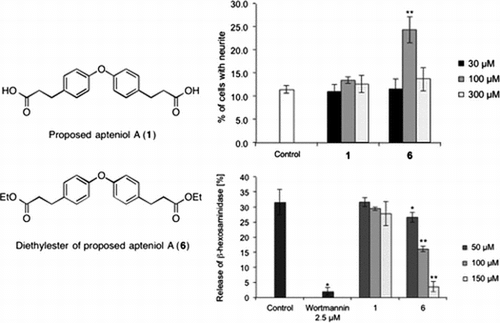
Table 1. NMR Data of 1 and reported apteniol A (in CD3OD).
Table 2. Effect of compound 1 on germination of L. sativa.
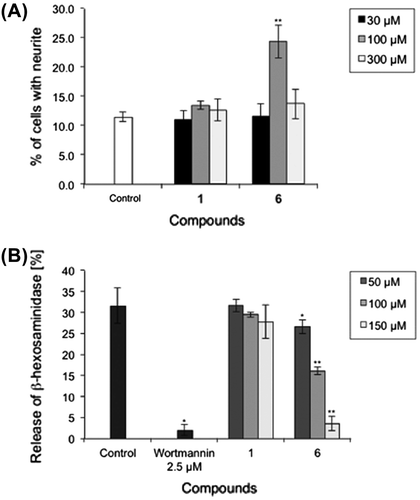
Fig. 1. Biological assay.
Notes: (A) Effects of proposed apteniol A and its derivatives on Bt2cAMP-induced neurite outgrowth in PC12 cells. PC12 cells were plated at 4.0 × 103 cells/well and cultured with indicated test compound concentrations in the presence of 0.5 mM Bt2cAMP. The extent of neurite outgrowth was measured after 24 h and expressed as the mean percentage of 300–400 cells from triplicate cultures. The bars represent SD. **p < 0.01 (Dunnett’s test) compared with the control (Bt2cAMP 0.5 mM only). (B) Inhibitory effects of proposed apteniol A and its derivatives on antigen-induced degranulation in RBL-2H3 cells. DNP-IgE sensitized RBL-2H3 cells were incubated with indicated test compound concentrations for 20 min and stimulated with DNP-HAS for 1 h. Released β-hexosaminidase was measured. Data correspond to means of triplicate cultures and the bars represent SD. *p < 0.05, **p < 0.01 (Dunnett’s test) compared with control.
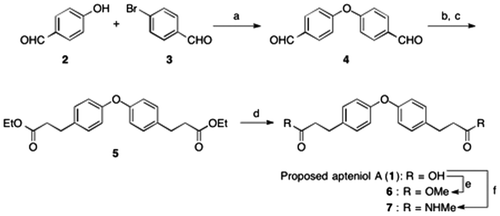
Scheme 1. Synthesis of proposed apteniol A and its derivatives.
Notes: Reagents and conditions: (a) CuI, N,N-dimethylglycine HCl salt, Cs2CO3, DMF, 100 °C (82%); (b) triethyl phosphonoacetate, NaH, benzene, rt (96%); (c) Pd/C, H2, MeOH, 50 °C (98%); (d) NaOH, THF/H2O, rt (62%); (e) H2SO4, MeOH, reflux (90%); (f) methylamine (40% in methanol), NaH, 100 °C (40%).