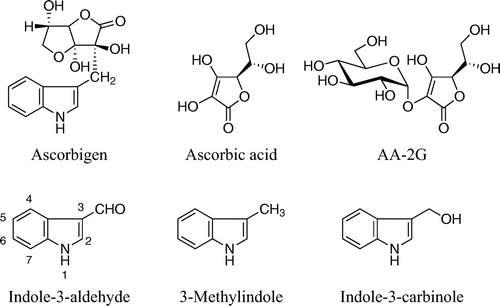
Figures & data
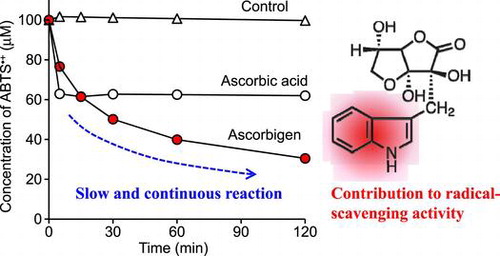
Fig. 2. Time courses of DPPH radical (A)-, galvinoxyl radical (B)-, and ABTS•+ (C)-scavenging reactions of ascorbigen, ascorbic acid, and AA-2G.
), ascorbic acid (○), and AA-2G (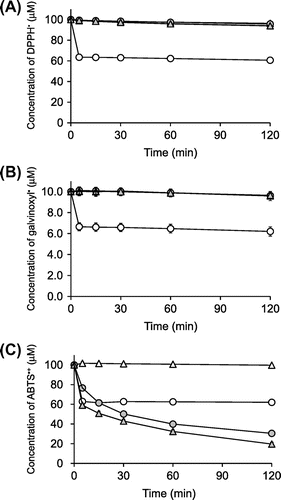
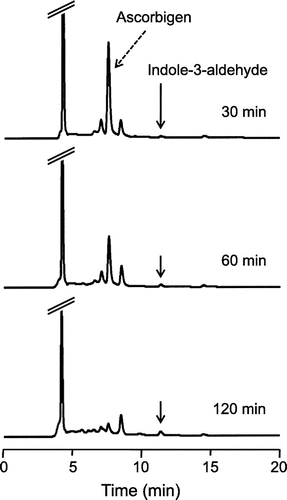
Fig. 3. HPLC chromatograms of the reaction product of ascorbigen with ABTS•+.
Notes: Ascorbigen (200 μM) and ABTS•+ (1.0 mM) were incubated in citrate buffer (50 mM, pH 6). At 30, 60, and 120 min, aliquots of the reaction mixture were withdrawn and analyzed by HPLC.
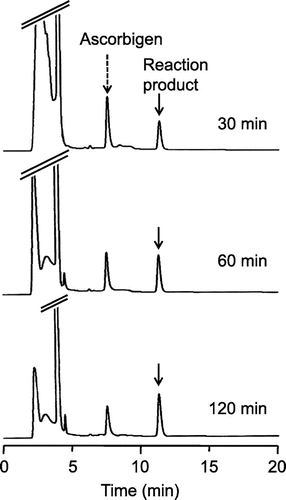
Fig. 4. Time courses of ABTS•+-scavenging reactions of ascorbigen, 3-methylindole, indole-3-aldehyde, and ascorbic acid.
), ascorbic acid (○), 3-methylindole (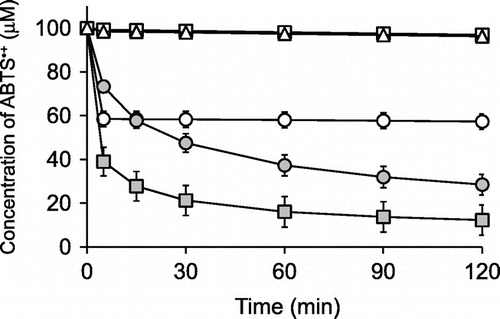
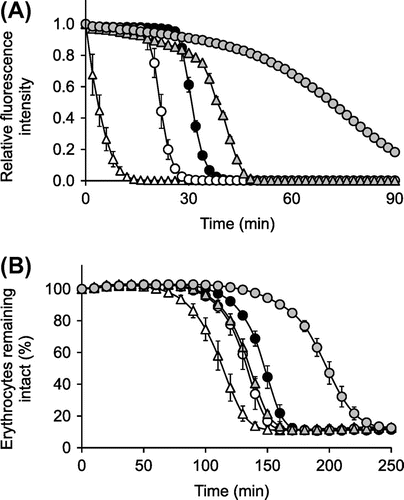
Fig. 5. ORAC assay (A) and OxHLIA (B) for ascorbigen, ascorbic acid, AA-2G, and Trolox.
Notes: ORAC assay (A) reaction mixtures containing ascorbigen (