Figures & data

Table 1. Phenolic content and DPPH scavenging activity of loquat leaves and LTE.
Fig. 1. The inhibitory effects of the extracts of loquat leaves and loquat tea on cell proliferation of HL-60 cells.
Notes: (A) The cells (1.5 × 104 cells/100 μL/well) were seeded into 96-well plates for 24 h, and then treated with 100 μg/mL of the loquat leaves water extract (L), loquat tea water extract (T), and C fraction separated from T by MCI gel with 50% EtOH (C) for another 24 h. (B) The cells were treated for 24 h with 25–100 μg/mL of C2 fraction, which was separated from C fraction by ODS gel with 20% MeOH. CTL cells were used as control cells without any treatment. MTT was added into medium for an additional 4 h. The survival of cells was detected by measuring the absorbance at a wavelength of 595 nm. The survival rate was expressed as the optical density ratio of the treatment to control. Each value represents the mean ± S.D. of triplicate cultures. Means with differently lettered superscripts differ significantly at the probability of p < 0.05.
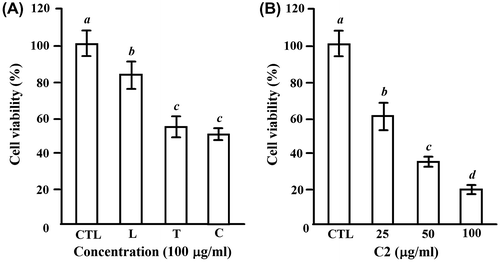
Fig. 2. C2 fraction induces morphology changes and DNA fragmentation of HL-60 cells.
Notes: HL-60 cells were exposed to 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h. Cell morphology was observed under an optical microscope (A). Then, the cells were harvested by centrifugation, and DNA was extracted as described in the Materials and methods section. The DNA fragments were separated on 2% agarose gel electrophoresis and visualized under ultraviolet light after staining with ethidium bromide. The 100 bp DNA Ladder One (Nacalai Tesque, Kyoto, Japan) served as a molecular marker (B).
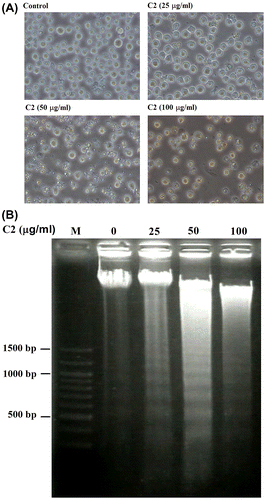
Fig. 3. C2 fraction-induced apoptosis involves the activation of caspase-8, caspase-9, and caspase-3 and inactivation of PARP.
Notes: (A) A dose-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) were treated with 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. (B) A time-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) were treated with 50 μg/mL of C2 fraction for 12 and 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. The result is a representative of triple experiments shown.
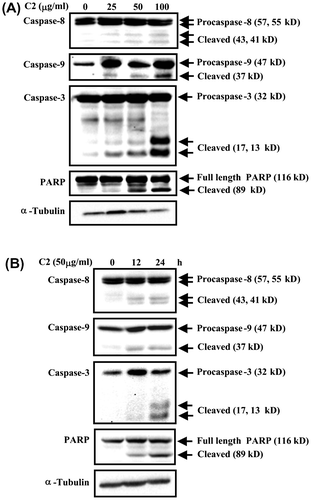
Fig. 4. The change in the protein levels of proapoptogenic Bax and antiapoptogenic Mcl-1 by C2 fraction.
Notes: (A) A dose-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) cells were treated with 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. (B) A time-dependent experiment. HL-60 (7.5 × 105/5 mL/6 cm dish) cells were treated with 50 μg/mL of C2 fraction for 12 and 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. Each value represents the mean ± S.D. of triplicate cultures. Means with differently lettered superscripts differ significantly at the probability of p < 0.05.
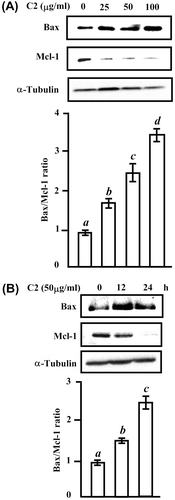
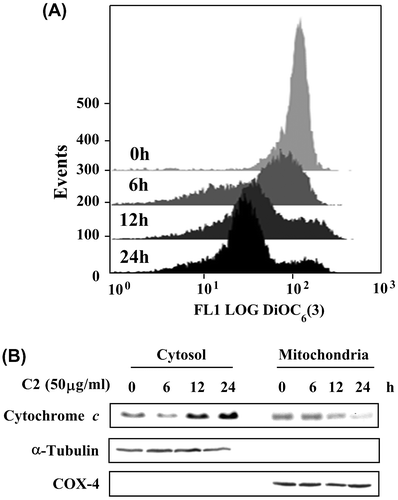
Fig. 5. The ΔΨm loss and cytochrome c release in HL-60 cells treated by C2 fraction.
Notes: (A) The ΔΨm loss. HL-60 (1 × 105/2 mL/6-wells plate) cells were treated with 50 μg/mL of C2 fraction for 0, 6, 12, and 24 h. The harvested cells were then incubated with 20 nM of DiOC6(3) for 30 min, followed by flow cytometric analysis as described under the Materials and methods section. (B) Cytochrome c release. HL-60 (7.5 × 105/5 mL/6 cm dish) cells were treated with 50 μg/mL of C2 fraction for 0, 6, 12, and 24 h. The harvested cells were fractionated into cytosolic and mitochondrial fractions as described under the Materials and methods section. Cytochrome c and the compartment-specific cytosolic α-tubulin or mitochondria COX-4 proteins were detected by Western blotting analysis with their respective antibodies.