Figures & data
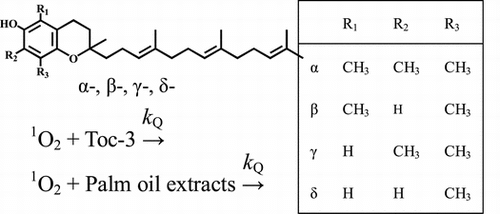
Fig. 1. Molecular structures of α-, β-, γ-, and δ-tocopherols (Tocs) and α-, β-, γ-, and δ-tocotrienols (Toc-3s), α- and γ-carboxyethyl-6-hydroxychromans (α- and γ-CEHCs), trolox, and 2-methyl-2-pentene.
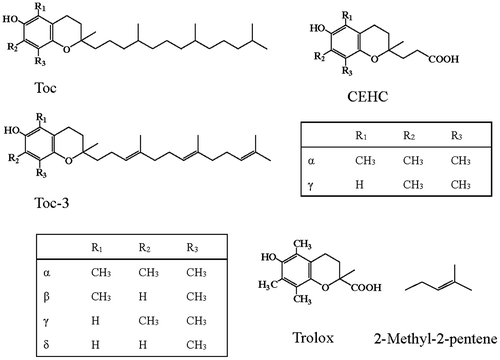
Table 1. (A) Contents of 8 vitamin E homologues (4 Tocs and 4 Toc-3s) included in palm oil extracts 1–5 and soybean extract 6 and (B) contents of 7 carotenoids included in palm oil extracts 1–5.
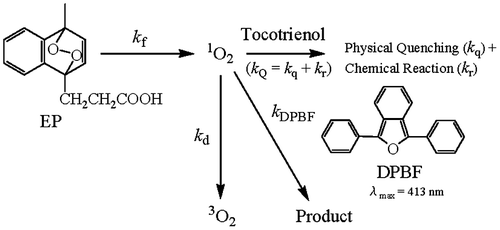
Fig. 2. Measurement of the second-order rate constant (kQ) for the reaction of γ-Toc-3 with 1O2.
Notes: (A) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of AOs (α-Toc or γ-Toc-3) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.76 × 10−5 M and [EP]t=0 = 4.41 × 10−4 M. The values of [α-Toc]t=0 and [γ-Toc-3]t=0 are shown in Fig. (A). (B) Plot of ln (absorbance) vs. t. (C) Plot of SBlank/Sγ-Toc-3 vs. [γ-Toc-3]. (D) Plot of t1/2γ-Toc-3/t1/2Blank vs. [γ-Toc-3].
![Fig. 2. Measurement of the second-order rate constant (kQ) for the reaction of γ-Toc-3 with 1O2.Notes: (A) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of AOs (α-Toc or γ-Toc-3) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.76 × 10−5 M and [EP]t=0 = 4.41 × 10−4 M. The values of [α-Toc]t=0 and [γ-Toc-3]t=0 are shown in Fig. 2(A). (B) Plot of ln (absorbance) vs. t. (C) Plot of SBlank/Sγ-Toc-3 vs. [γ-Toc-3]. (D) Plot of t1/2γ-Toc-3/t1/2Blank vs. [γ-Toc-3].](/cms/asset/392f38cc-eab1-4133-9f97-d45c37f499ea/tbbb_a_943653_f0002_b.gif)
Table 2. UV–vis absorption maxima (λmax) and molar extinction coefficients (εmax) of the antioxidants (tocopherols, tocotrienols, CEHCs, and Trolox) in ethanol/chloroform/D2O and ethanol, and peak oxidation potentials (Ep).
Table 3. The kQAO (S) and kQAO (t1/2) values for 8 vitamin E homologues, α- and γ-CEHCs, trolox, and 2-Methyl-2-pentene (AOs) in ethanol/chloroform/D2O solution at 35.0 °C, relative rate constants (kQAO (S)/kQα-Toc (S)), relative SOAC values, and ratio of the rate constants (kQToc−3 (S)/kQToc (S)).
Fig. 3. Measurement of the second-order rate constant (kQ) for the reaction of palm oil extract 1 with 1O2.
Notes: (A) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of AOs (α-tocopherol and palm oil extract 1) in ethanol/chloroform/D2O at 35 °C. [DPBF] = 6.29 × 10−5 M and [EP] = 4.20 × 10−4 M. The values of [α-Toc] and [Extract 1] are shown in Fig. (B). (B) Change in absorbance of DPBF, where the correction of baseline due to palm oil extract 1 was performed (see text). (C) Plot of ln (absorbance) vs. t. (D) Plot of SBlank/SExtract 1 vs. [Extract 1]. (E) Plot of t1/2Extract 1/t1/2Blank vs. [Extract 1].
![Fig. 3. Measurement of the second-order rate constant (kQ) for the reaction of palm oil extract 1 with 1O2.Notes: (A) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of AOs (α-tocopherol and palm oil extract 1) in ethanol/chloroform/D2O at 35 °C. [DPBF] = 6.29 × 10−5 M and [EP] = 4.20 × 10−4 M. The values of [α-Toc] and [Extract 1] are shown in Fig. 3(B). (B) Change in absorbance of DPBF, where the correction of baseline due to palm oil extract 1 was performed (see text). (C) Plot of ln (absorbance) vs. t. (D) Plot of SBlank/SExtract 1 vs. [Extract 1]. (E) Plot of t1/2Extract 1/t1/2Blank vs. [Extract 1].](/cms/asset/b2ac13d1-15c1-449f-b113-2a9fdb22ae03/tbbb_a_943653_f0003_b.gif)
Table 4. The kQExtract (S) and kQExtract (t1/2) values for palm oil extracts 1–5 and soybean extract 6 in ethanol/chloroform/D2O solution at 35.0 °C, relative rate constants (kQExtract (S)/kQα-Toc (S)), and relative SOAC values.
Table 5. Comparison between observed and calculated 1O2-quenching rates (kQExtract 5 (S) (Obsd.) and Σ kQAO-i (S) × ([AO-i]/100) (Calcd.)) for palm oil extract 5 in ethanol/chloroform/D2O solution.
Table 6. Comparison between observed and calculated 1O2-quenching rates (kQExtract (S) (Obsd.) and kQExtract (S) (Calcd.)) for palm oil extracts 1–5 and soybean extract 6 in ethanol/chloroform/D2O solution, ratio of the rate constants (kQExtract (S) (Obsd.)/kQExtract (S) (Calcd.)), and total wt% of Tocs and Toc-3s.
Table 7. The kQAO (S) and relative SOAC values for 11 tocopherol derivatives in ethanol/chloroform/D2O and ethanol solutions at 35.0 °C.
Fig. 4. Plot of kQ (S) (EtOH) vs. kQ (S) (mixed solvent) for (A) four Tocs (○), four Toc-3s (□), two CEHCs (Δ), and trolox (●); (B) α-Toc (○), palm oil extracts 1–5 (□), and soybean extract 6 (Δ); and (C) four Tocs (○), four Toc-3s (□), two CEHCs (Δ), trolox (●), extracts 1–5 (■), and extract 6 (▲).
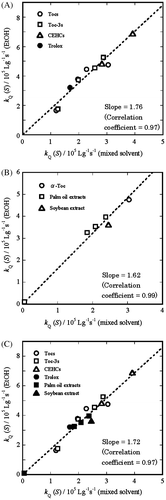
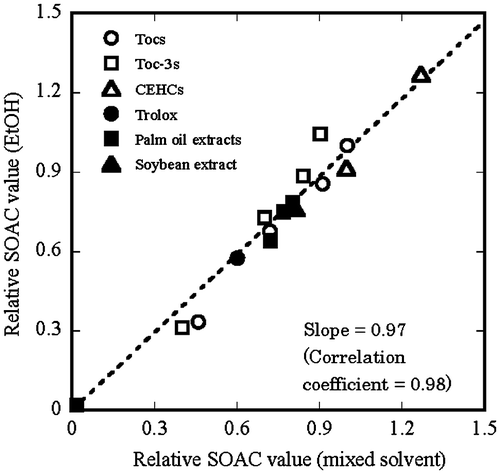
Fig. 5. Relative SOAC value (EtOH) vs. relative SOAC value (mixed solvent) for four Tocs (○), four Toc-3s (□), two CEHCs (Δ), trolox (●), extracts 1–5 (■), and extract 6 (▲).