Figures & data
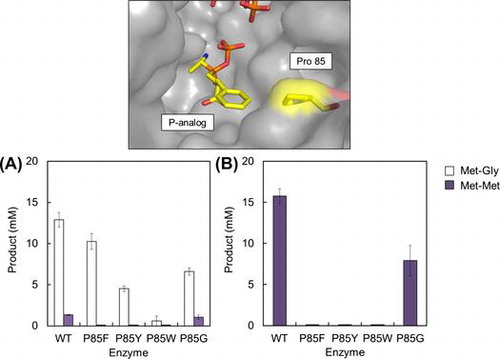
Fig. 1. Alignment of primary structures of Lals.

Fig. 2. Structure of Ywfe in complex with the phosphinate L-alanyl-L-phenylalanine analog (P-analog) Citation22) is superimposed onto the BL00235 crystal structure (PDB code, 3VOT).Citation21)
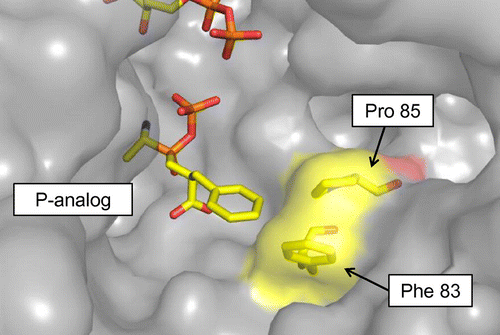
Fig. 3. Synthesis of Met-Gly (white bars) and Met-Met (dark bars) by TabS and BL00235.
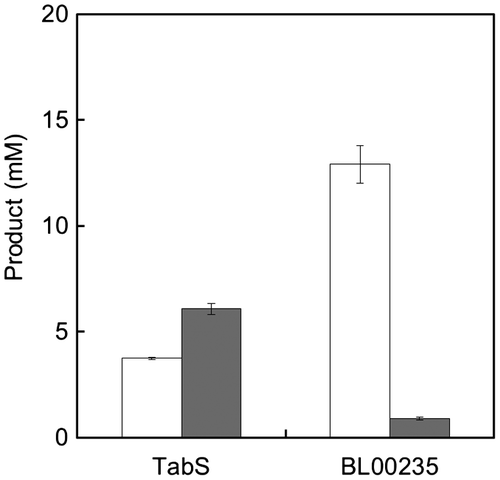
Fig. 4. Synthesis of Met-Gly (white bars) or Met-Met (dark bars) by the wild-type BL00235 or mutants.
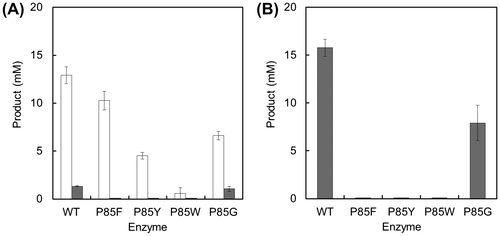
Fig. 5. Kinetic analysis of Met-Gly-synthesizing reaction by the wild-type BL00235 or the P85F mutant.
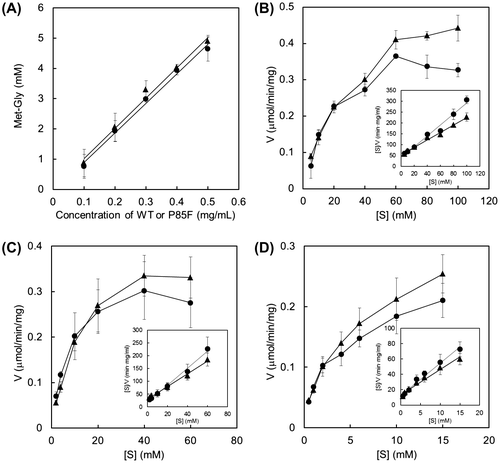
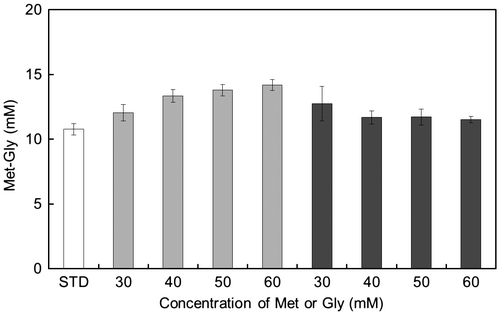
Fig. 6. Effect of substrate concentration on Met-Gly synthesis by the P85F mutant.