Figures & data
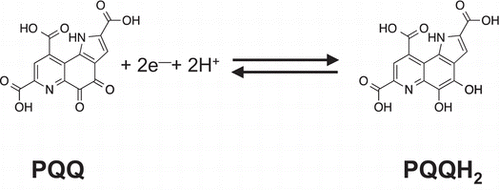
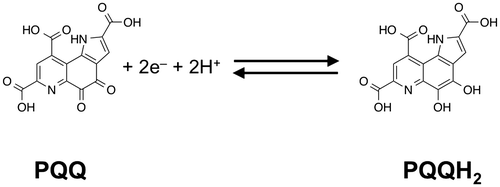
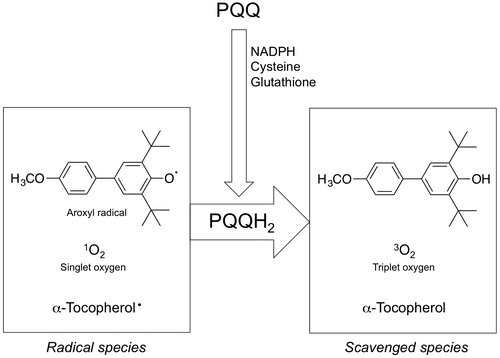
Fig. 2. Proposed mechanism for the ligand-independent activation of epidermal growth factor receptor (EGFR) signaling through redox cycling of pyrroloquinoline quinone (PQQ). PQQ undergoes redox cycling in the presence of reductants, such as ascorbate and glutathione, and then produces and H2O2. The generated H2O2 inactivates protein tyrosine phosphatase 1B (PTP1B) via the oxidation of catalytic cysteinyl thiol (Cys-215) to the corresponding sulfenic acid (–SOH), sulfinic acid (–SO2H), and sulfonic acid (–SO3H). The inhibition of PTP1B evokes the EGF-independent activation (tyrosine phosphorylation) of EGFR and subsequent activation (serine/threonine phosphorylation) of ERK 1/2.
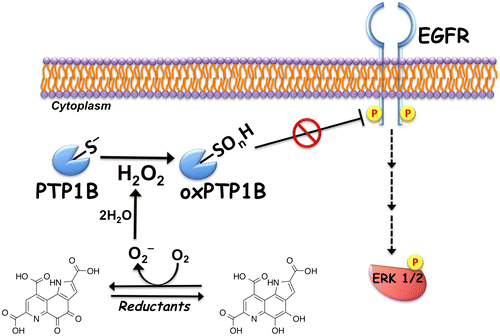
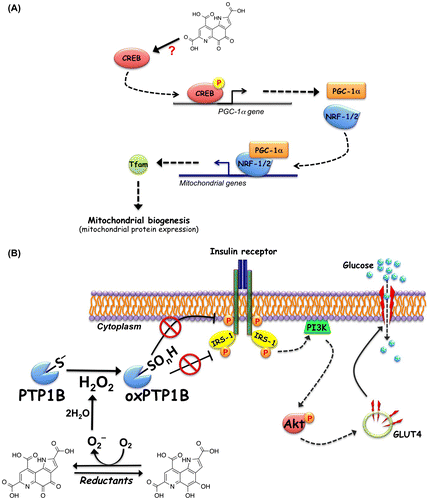
Fig. 3. Pyrroloquinoline quinone (PQQ)-induced activation of cAMP-responsive element-binding protein (CREB)-peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) and insulin signaling. (A) Proposed mechanism for PQQ-induced activation of CREB-PGC-1α signaling pathway. PQQ stimulates the phosphorylation and activation of CREB and enhances PGC-1α expression. Increased PGC-1α binds to and coactivates the transcriptional function of nuclear respiratory factor (NRF)-1/2 on the mitochondrial transcription factor A (Tfam) promoter. Tfam plays a crucial role in regulating mtDNA amplification and mitochondrial biogenesis. (B) Proposed mechanism for the ligand-independent activation of insulin signaling through redox cycling of PQQ. PQQ inhibits protein tyrosine phosphatase 1B (PTP1B) to oxidatively modify the catalytic cysteine through its redox cycling activity. The inhibition of PTP1B evokes the insulin-independent activation (tyrosine phosphorylation) of the insulin receptor (IR) and subsequent phosphorylation of insulin receptor substrate-1 (IRS-1) and Akt. Phosphorylated Akt stimulates translocation of glucose transporter 4 (GLUT4) to the plasma membrane, resulting in increased cellular glucose uptake.