Figures & data
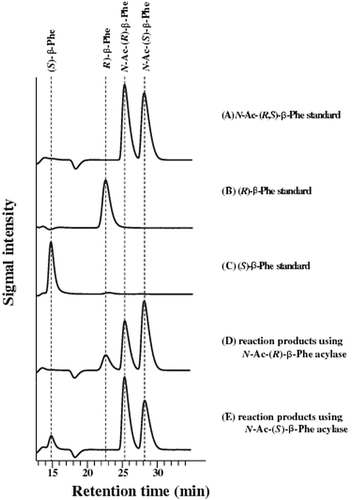
Fig. 1. Separation of N-Ac-(R)- and (S)-β-Phe acylase activities using a Phenyl Sepharose column.
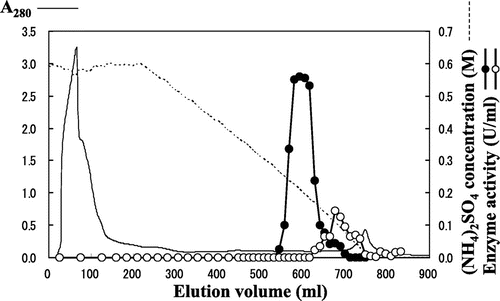
Fig. 2. SDS-PAGE analysis of the purified (A) N-Ac-(R)-β-Phe acylase and (B) N-Ac-(S)-β-Phe acylase.
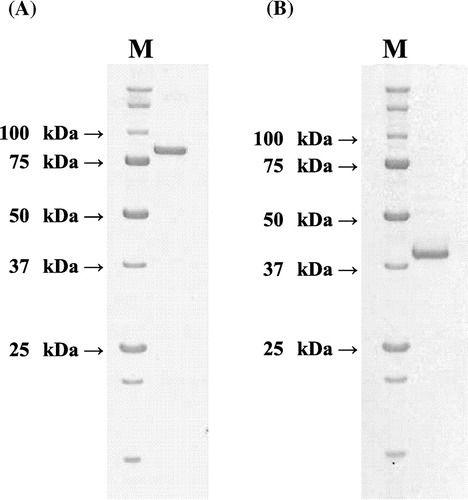
Table 1. Purification of N-Ac-(R)-β-Phe acylase and N-Ac-(S)-β-Phe acylase.
Fig. 3. Enantioselectivity of purified acylases.
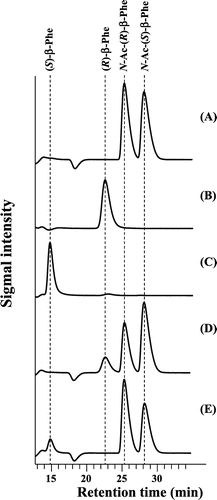
Fig. 4. Genetic organizations around the gene encoding (A) N-Ac-(R)-β-Phe acylase and (B) N-Ac-(S)-β-Phe acylase.
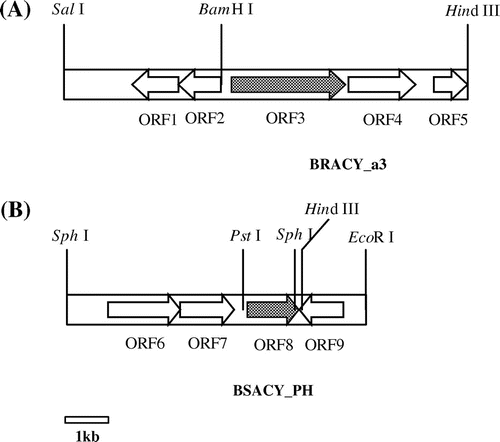
Table 2. Location and properties of sequenced genes and proteins predicted to be encoded by those genes.
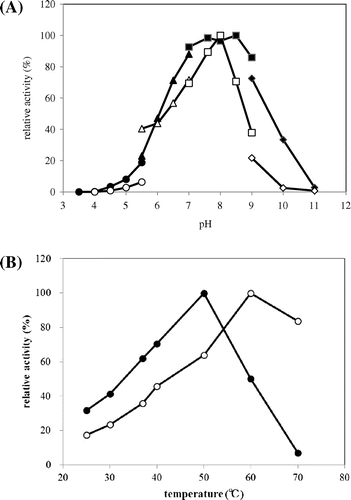
Fig. 5. Optimum pH (A) and temperature (B) of N-Ac-(R)-β-Phe acylase and N-Ac-(S)-β-Phe acylase.