Figures & data
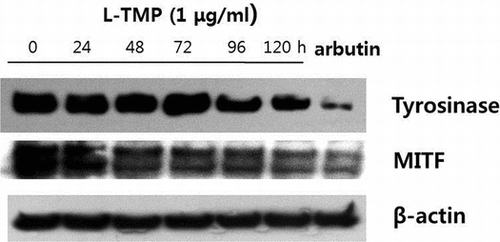
Fig. 2. Effects of L-TMP on the proliferation of NHMs.
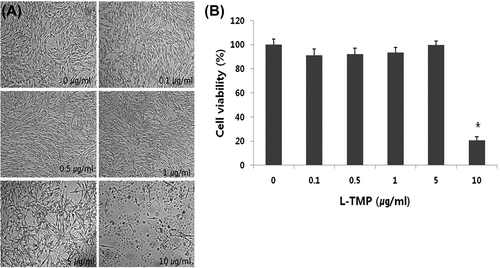
Fig. 3. Effects of L-TMP on melanogenesis.
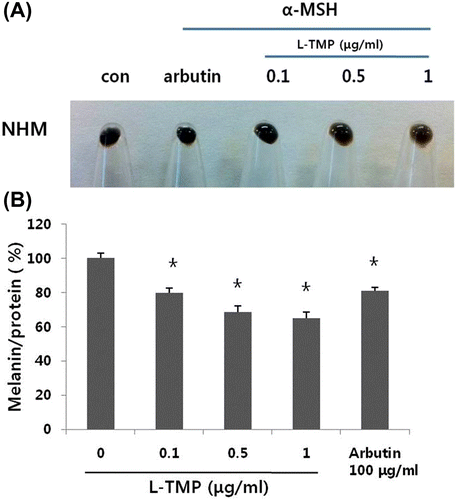
Fig. 4. Effects of L-TMP on tyrosinase activity. L-TMP suppressed tyrosinase activity in NHMs.
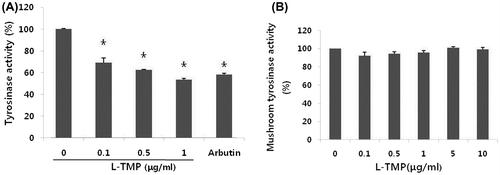
Fig. 5. Effects of L-TMP on melanogenic protein expression and the ERK, p38 and PKC pathways in NHMs.
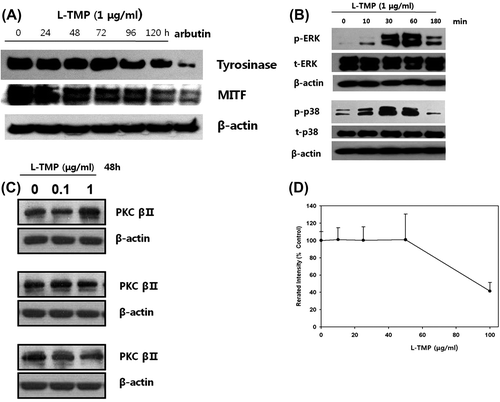
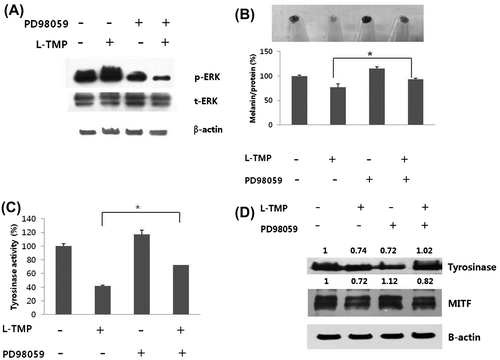
Fig. 6. Effects of ERK inhibitor (PD98059) on the L-TMP-induced downregulation of melanogenesis.