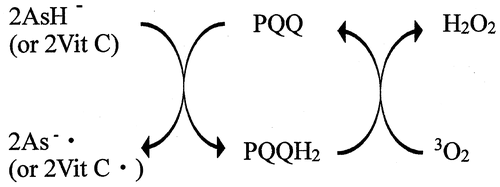
Figures & data
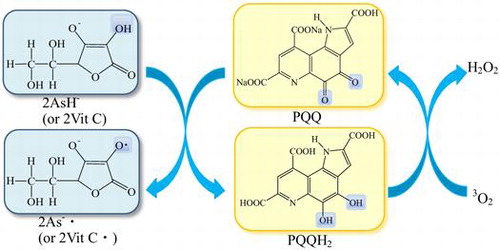
Fig. 1. Molecular structures of PQQNa2, PQQH2, vitamin C (ascorbic acid, AsH2), and ascorbate monoanion (AsH−).
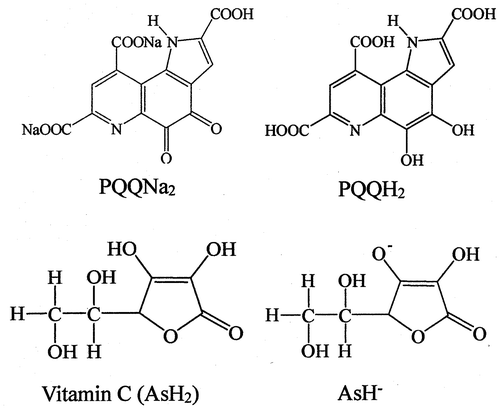
Fig. 2. The UV–vis absorption spectra of PQQNa2 (–––), PQQH2 (·····), and Vit C (AsH−) (- - -) with the same concentrations of 4.45 × 10−5 M in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0°.
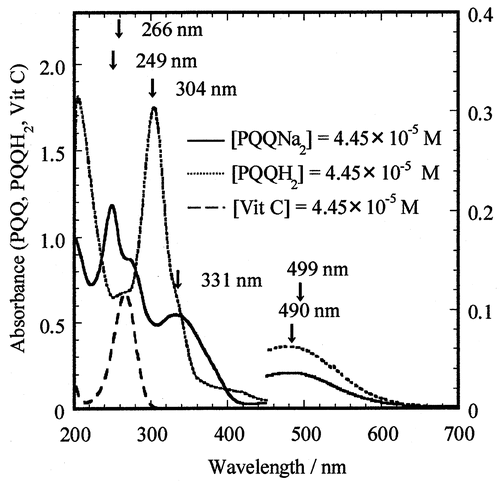
Table 1. Values of UV–vis absorption maxima (
 ) and molar extinction coefficients (
) and molar extinction coefficients (
 ) for PQQNa2, PQQH2, and Vit C in 0.05 M phosphate-buffered solution (pH 7.4).
) for PQQNa2, PQQH2, and Vit C in 0.05 M phosphate-buffered solution (pH 7.4).
Fig. 3. Changes in the absorption spectrum of PQQNa2 and PQQH2 during the reaction of PQQNa2 with Vit C in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0 °C.
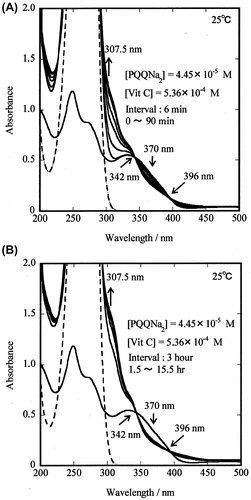
Fig. 4. Formation of PQQH2 due to the reaction of PQQNa2 with Vit C, and decay of PQQH2 in the presence of air.
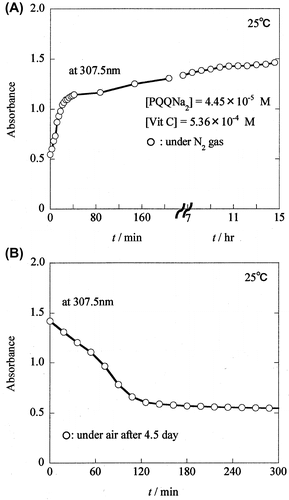
Fig. 5. Formation of PQQH2 due to the reaction of PQQNa2 with Vit C, and decay of PQQH2 in the presence of air.
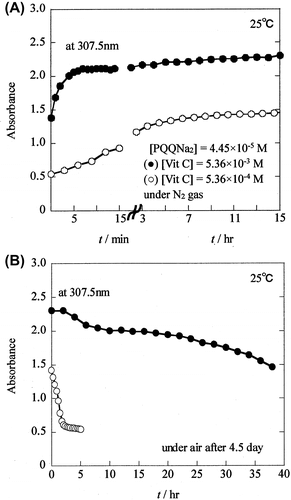
Fig. 6. PQQH2 solution produced due to the reaction of PQQNa2 ([PQQNa2]t = 0 = 4.45 × 10−5 M) with Vit C ([Vit C]t = 0 = 5.36 × 10−4 M) was exposed to air, after keeping the solution under nitrogen atmosphere for 4.5 day.
![Fig. 6. PQQH2 solution produced due to the reaction of PQQNa2 ([PQQNa2]t = 0 = 4.45 × 10−5 M) with Vit C ([Vit C]t = 0 = 5.36 × 10−4 M) was exposed to air, after keeping the solution under nitrogen atmosphere for 4.5 day.](/cms/asset/ec26a0ea-6ba5-493d-b1aa-a733138ebad3/tbbb_a_1072462_f0006_b.gif)