Figures & data
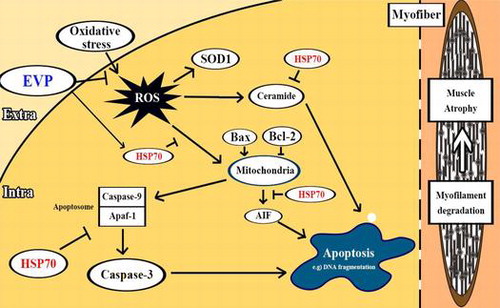
Fig. 1. Effects of intraperitoneal injection of EVP on disuse muscle atrophy by sciatic denervation in mice.
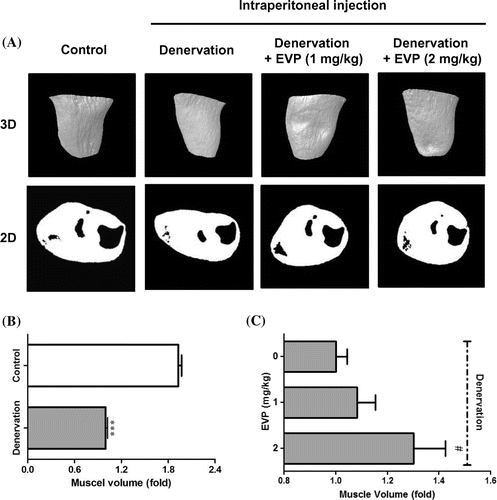
Fig. 2. Effects of EVP on substances and proteins relating to the pathway between ROS and apoptosis in muscle of sciatic-denervated mice.
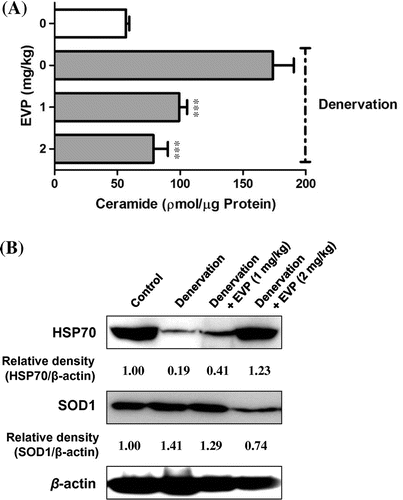
Fig. 3. Effect of EVP on H2O2-induced oxidative stress in C2C12 myoblasts.
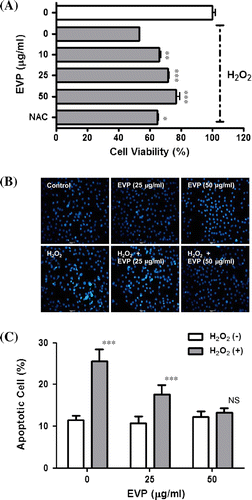
Fig. 4. Effect of EVP on ceramide level in H2O2-induced oxidative stress in C2C12 myoblasts.
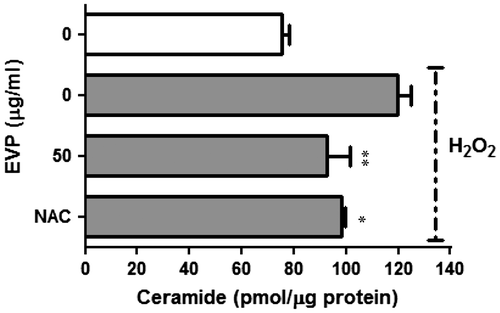
Fig. 5. Effect of EVP on oxidative stress induced by ROS in C2C12 myoblasts.
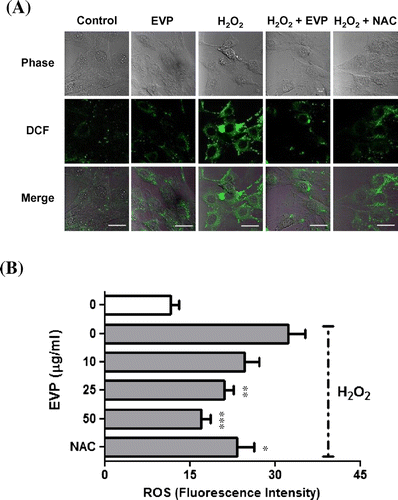
Fig. 6. Effect of EVP on SOD1 expression in H2O2-induced oxidative stress in C2C12 myoblasts.
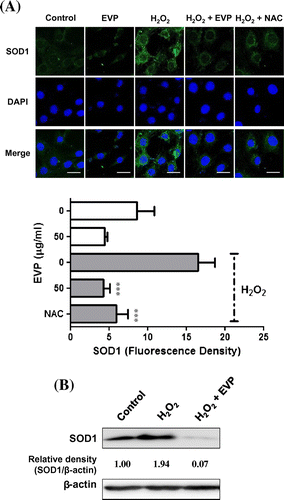
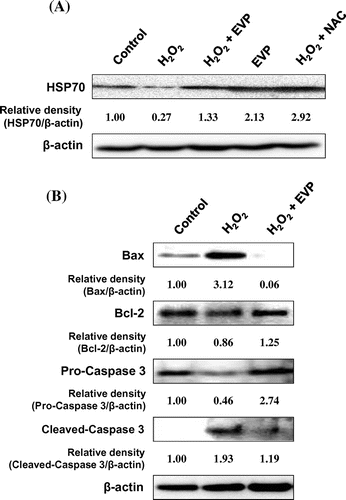
Fig. 7. Effect of EVP on HSP70 expression in H2O2-induced oxidative stress in C2C12 myoblasts.