Figures & data
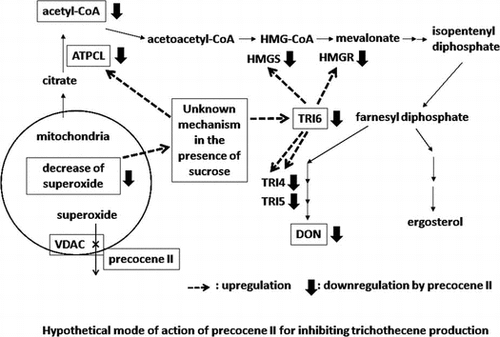
Fig. 1. Structures of aflatoxins and aflatoxin production inhibitors from plant constituents.
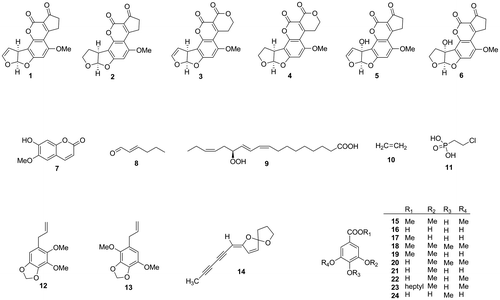
Fig. 2. Aflatoxin production inhibitors from microbial metabolites and related compounds.
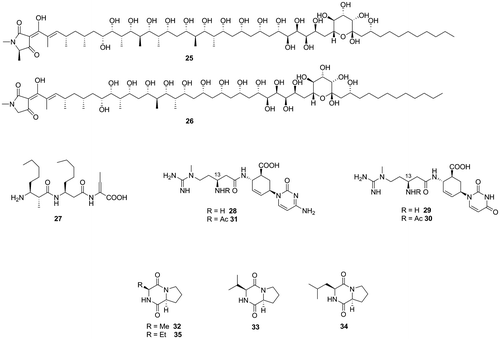
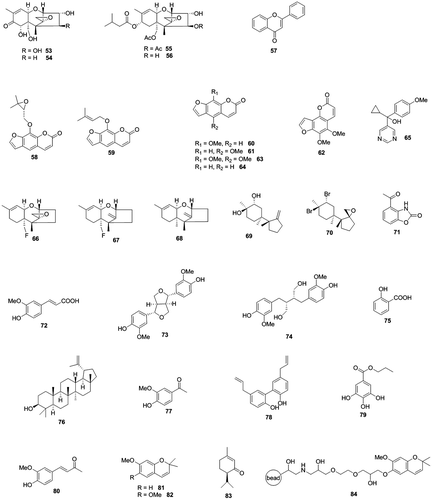
Fig. 3. Aflatoxin production inhibitors from pesticides, respiration inhibitors, and synthetic bioactive compounds.
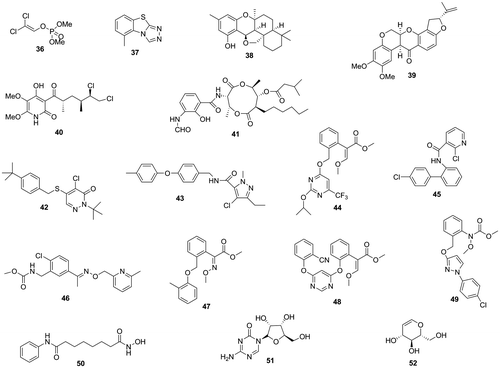
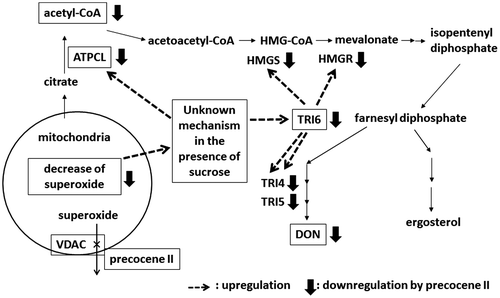
Fig. 4. Trichothecenes, trichothecene production inhibitors, and precocene II-immobilized magnet bead (84).