Figures & data
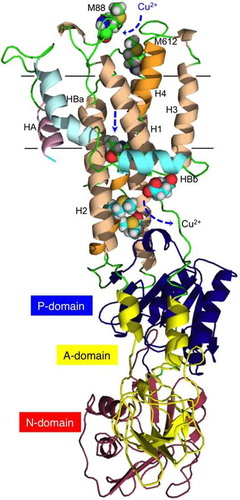
Table 1. Effects of dl-penicillamine on generation time in log phase B. subtilis 168.
Fig. 1. Effects of metals and 0.1% dl-penicillamine on growth of B. subtilis 168.
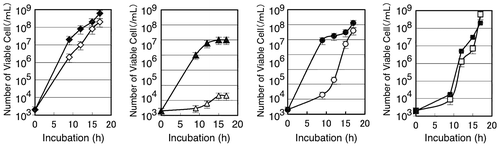
Table 2. B. subtilis 168 genes responding to 0.1% dl-penicillamineTable Footnotea.
Fig. 2. Characterization of the zosA mutant.
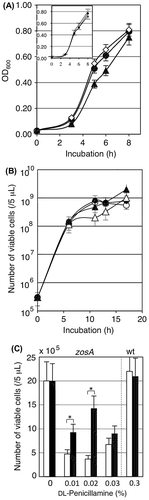
Fig. 3. The wild-type strain (open symbols) and zosA mutant (closed symbols) were grown overnight on LB media containing either CuSO4 (triangles) or ZnSO4 (circles) at the concentrations designated on the x-axis.
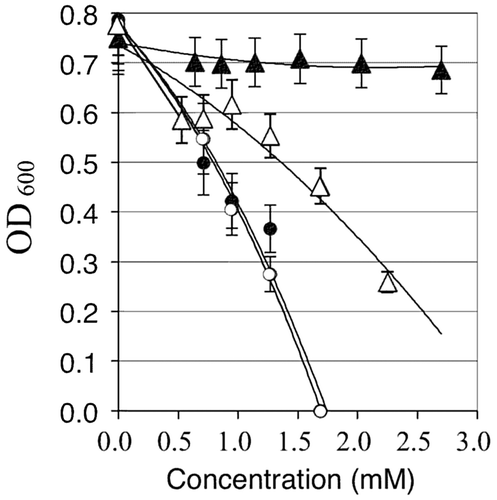
Fig. 4. Effect of zosA mutation on the metal tolerance of B. subtilis 168.
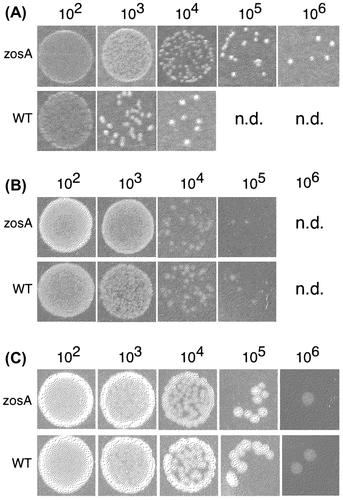
Fig. 5. Effect of the csoR-copZA deletion on Cu-sensitivity of B. subtilis 168.
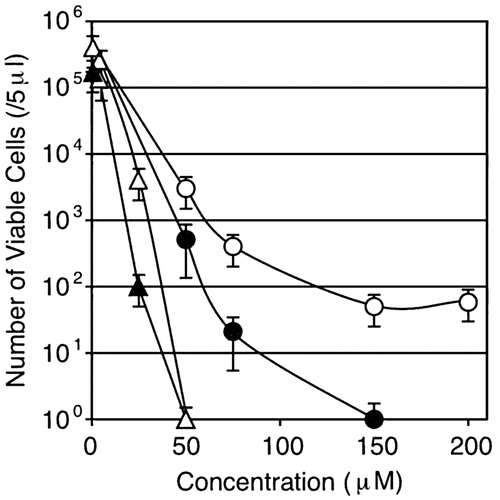
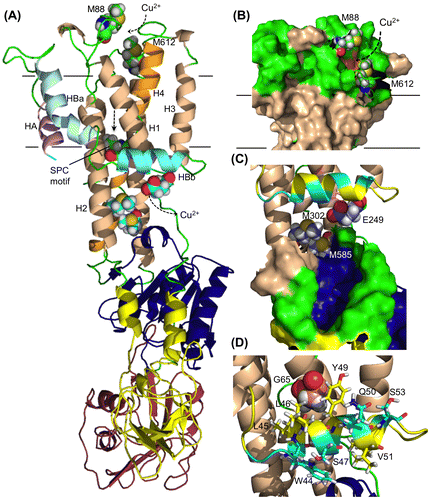
Fig. 6 Modeling of ZosA.