Figures & data
Fig. 1. Electrophoretic analysis of PYG.
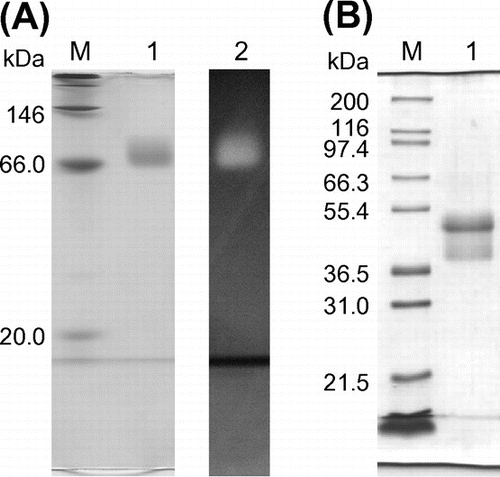
Fig. 2. MALDI-TOF-MS analysis of PYG tryptic peptides.
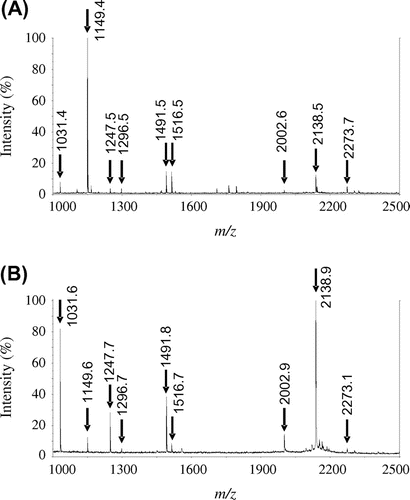
Fig. 3. Effect of various salts on enzyme activity.
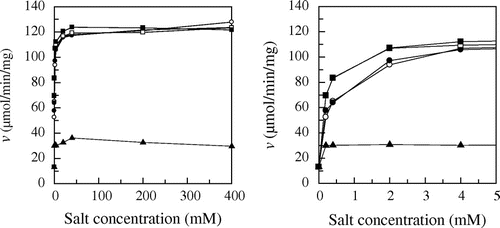
Table 1. PYG purification summary.
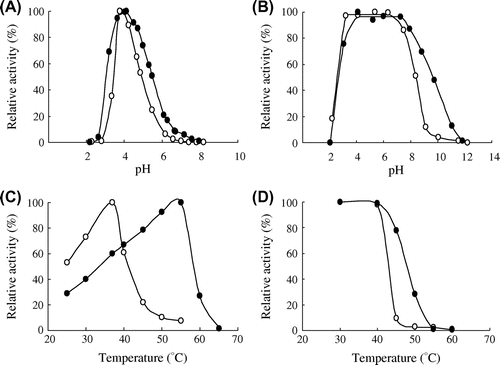
Fig. 4. Effect of NaCl on the pH–activity profile (A), the pH–stability (B), the temperature–activity profile (C), and the thermal stability (D) of PYG in the absence (○) and presence (●) of 300 mM NaCl.