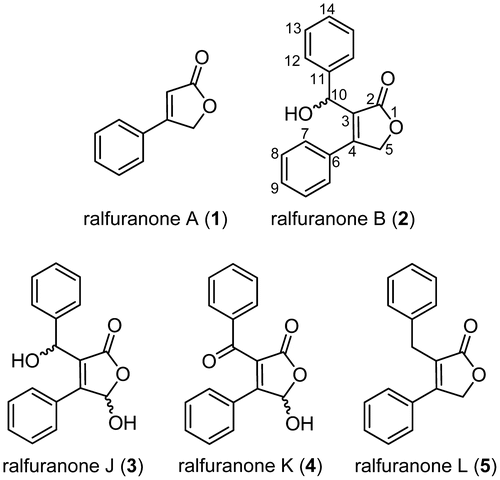
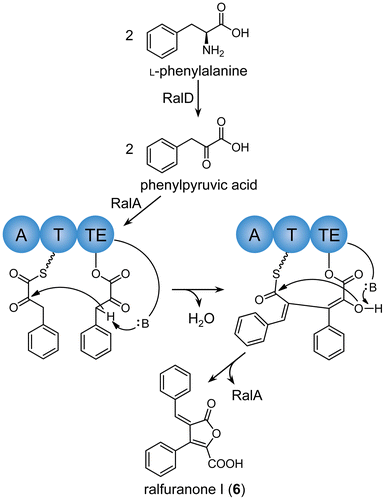
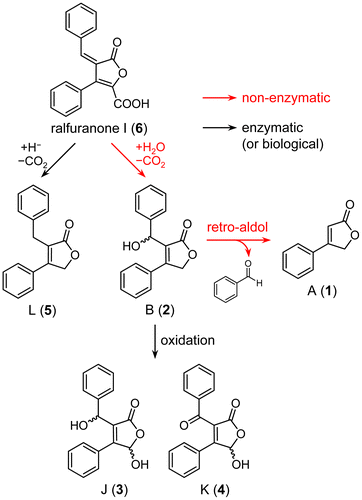
Figures & data
Fig. 2. HPLC chromatograms of EtOAc extracts from ∆ralA cultures fed with 2.5 μM (A) and 200 μM ralfuranone I (6) (B) and MGRLS medium fed with 2.5 μM ralfuranone I (6) (C). The peaks of ralfuranones were marked with arrowheads.
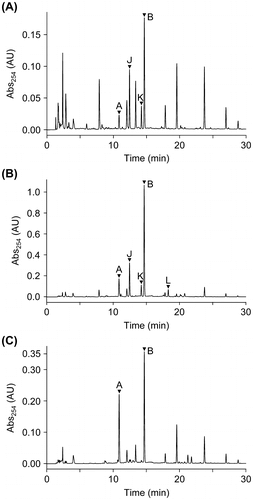
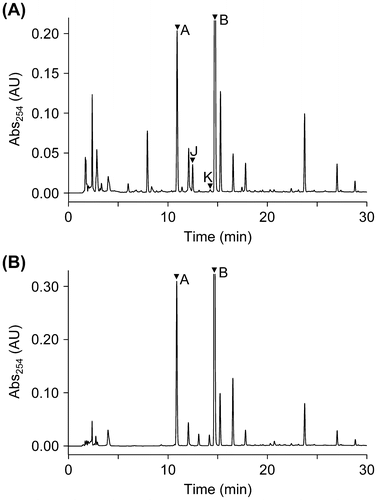
Fig. 3. HPLC chromatograms of EtOAc extracts from ∆ralA cultures (A) and MGRLS medium fed with 10 μM ralfuranone B (2) (B). The peaks of ralfuranones were marked with arrowheads.