Figures & data
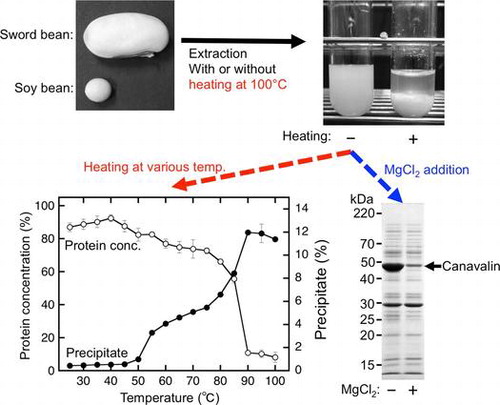
Fig. 1. Size transition of sword beans and weight transition of absorbed water during soaking.
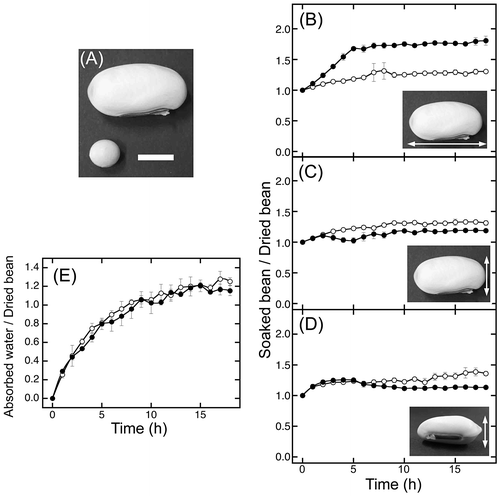
Table 1. Comparison of the size and weight between dried beans.
Fig. 2. Protein extraction from sword beans.
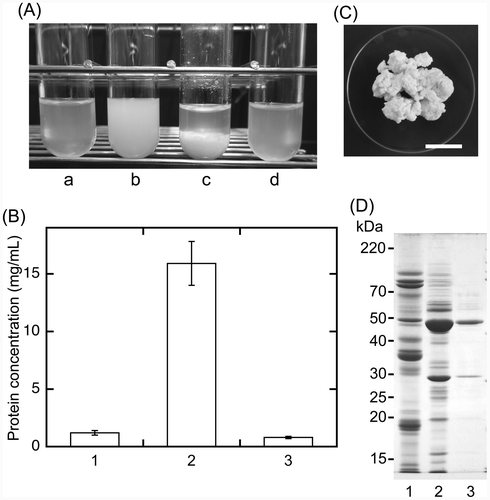
Fig. 3. Temperature-dependent precipitate formation of sword bean proteins.
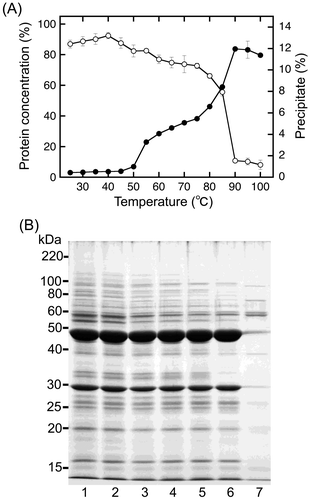
Fig. 4. Magnesium chloride-dependent precipitation of sword bean proteins.
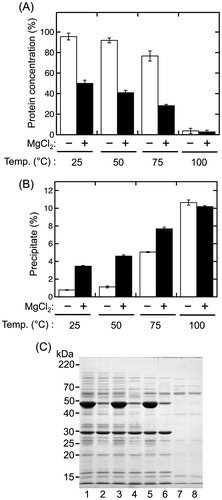
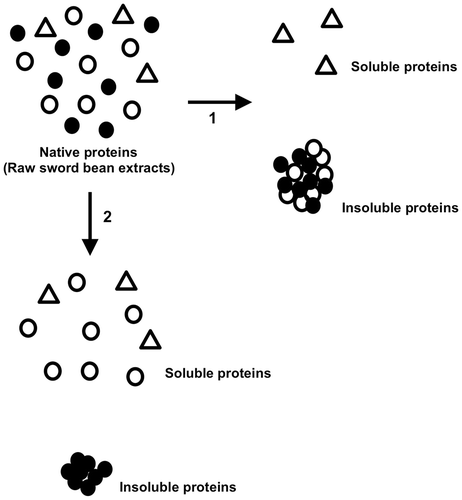
Fig. 5. Schematic model for the precipitation of sword bean proteins under different conditions.