Figures & data
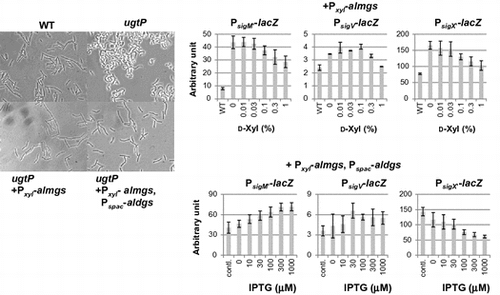
Table 1. Strains and plasmids used in this study.
Table 2. Primers used in this study.
Fig. 1. pgcA and gtaB disruptants showed the same phenotype as the ugtP mutant.
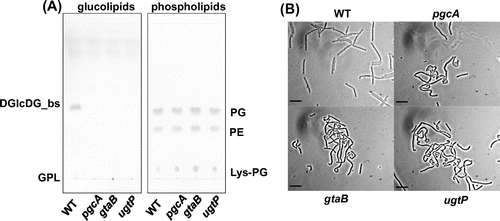
Fig. 2. The abnormal morphology of the B. subtilis ugtP mutant was suppressed when heterologous MGlcDG synthase was expressed.
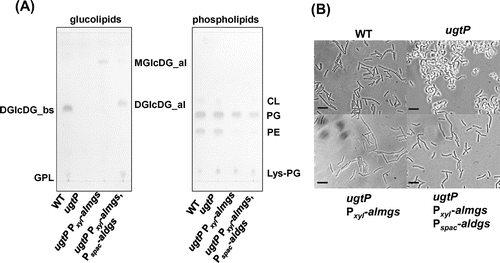
Fig. 3. Effect of heterologous glucolipids on activitiy of ECF sigmas in the B. subtilis ugtP mutant.
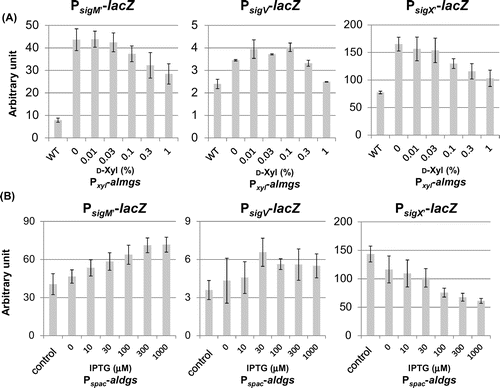
Fig. 4. Effect of heterologous glucolipids production on activitiy of SigM and SigV in the B. subtilis wild type.
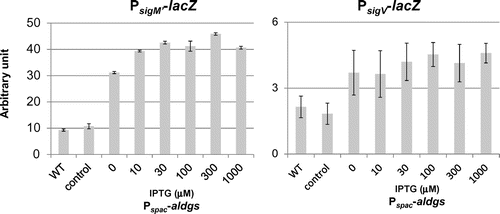
Fig. 5. Effect of ugtPH18A expression and Mg2+ addition on activities of ECF sigmas in B. subtilis ugtP mutant.
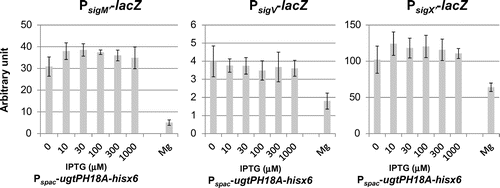
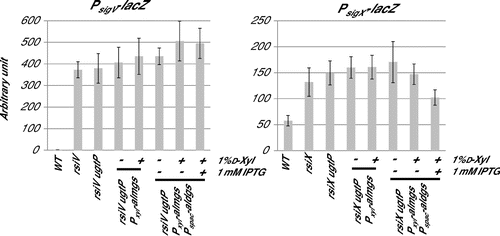
Fig. 6. Effect of expression of heterologous glucolipids on activitiy of SigV and SigX in the B. subtilis without their anit-sigma factors.