Figures & data
Table 1. Chitinase and CERK candidate genes in P. patens.
Fig. 1. Schematic representation of primary structure of chitinases from P. patens.
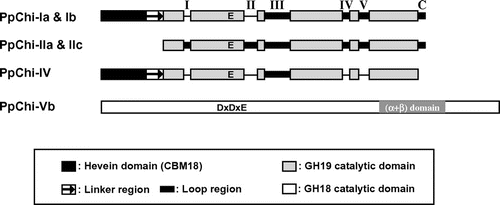
Fig. 2. Expression analysis of chitinase genes by RT-qPCR.
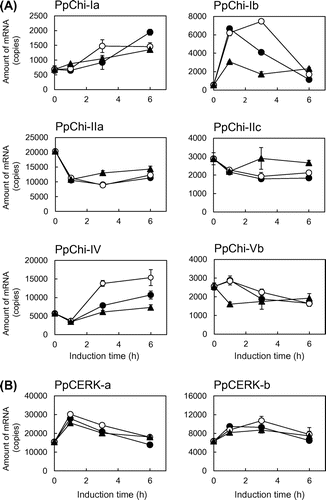
Fig. 3. SDS–PAGE of recombinant PpChis.
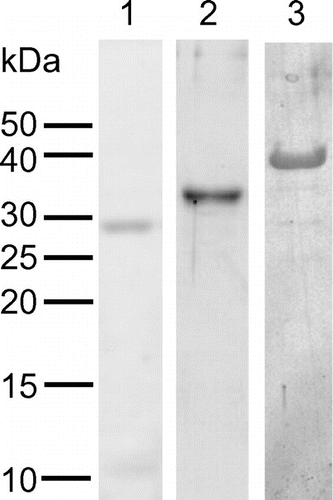
Fig. 4. Effect of pH and temperature on the chitinase activity.
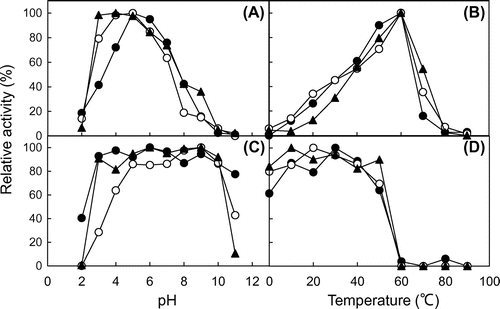
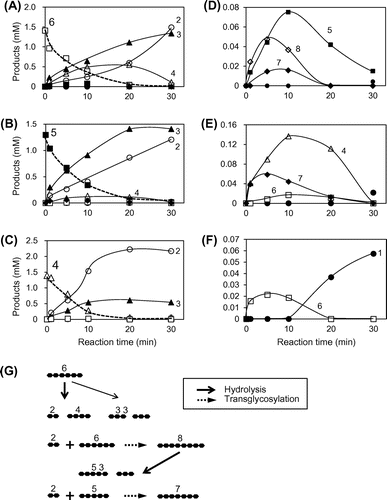
Fig. 5. Time-courses of the PpChi-Ia and -IV catalyzed reaction toward (GlcNAc)6–4. Time-course of the reaction products of (GlcNAc)6–4 by PpChi-Ia (A, B, and C) and PpChi-IV (D, E, and F).
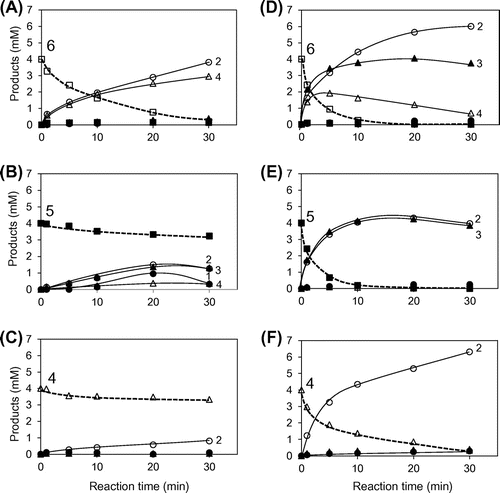
Fig. 6. Time courses of (GlcNAc)6–4 hydrolysis and transglycosylation catalyzed by PpChi-Vb.