Figures & data
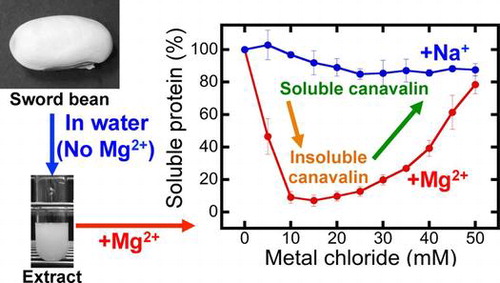
Fig. 1. Effects of magnesium chloride (MgCl2) and sodium chloride (NaCl) concentrations on canavalin solubility.
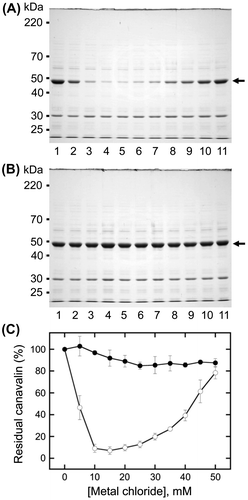
Fig. 2. Effects of high sodium chloride (NaCl) concentrations on canavalin solubility.
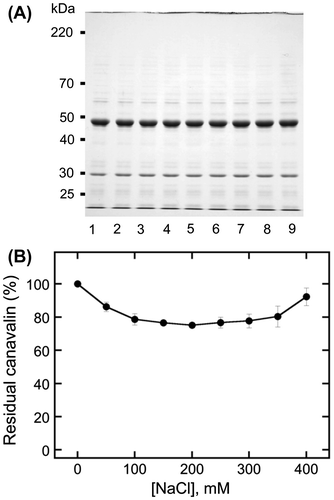
Fig. 3. Analysis of canavalin solubility induced by magnesium chloride (MgCl2).
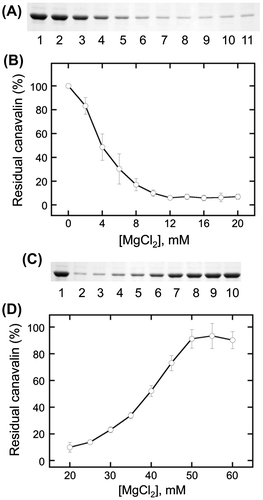
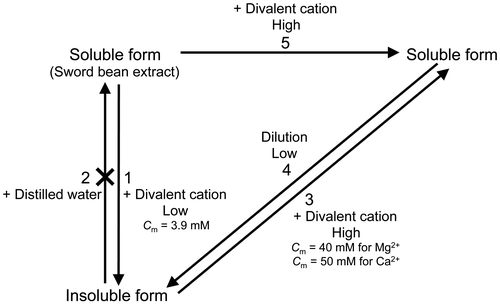
Table 1. Midpoint concentrations for the insolbulization and solubilization of canavalin.
Fig. 4. Effects of calcium chloride (CaCl2) concentration on canavalin solubility.
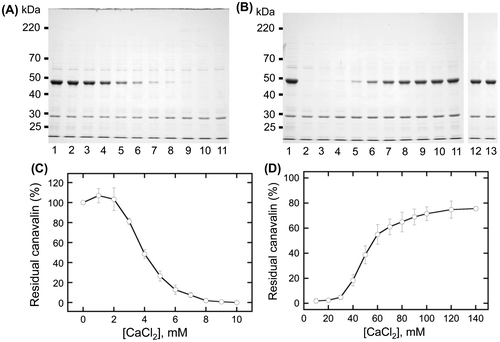
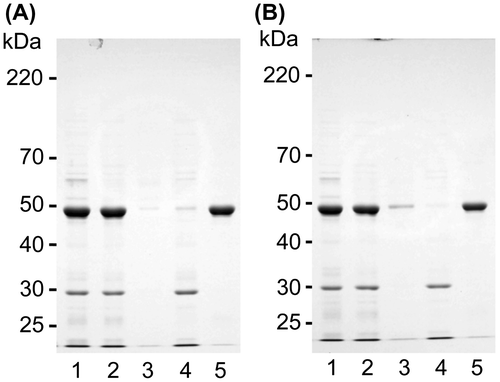
Fig. 5. Solubility changes to insolubilized canavalin.
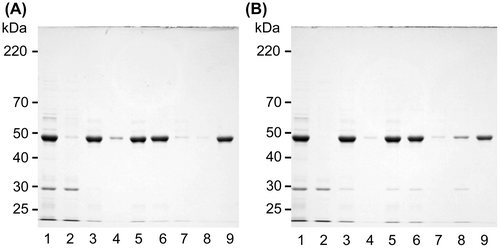
Fig. 6. Solubility changes to solubilized canavalin.