Figures & data
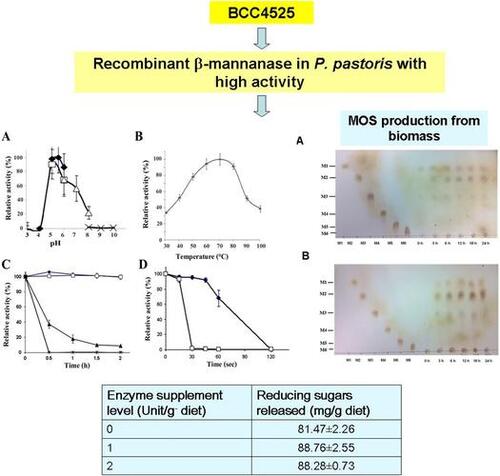
Table 1. Purification of the recombinant β-mannanase from BCC4525.
Fig. 1. Expression of the recombinant MANF3 in KM71 P. pastoris and its characterization. (A) Effect of pH on recombinant BCC4525 β-mannanase activity was determined at different pH values by incubating the enzyme at 70 °C for 10 min in the following buffers; sodium acetate buffer pH 3–6 (closed diamond, ![]()
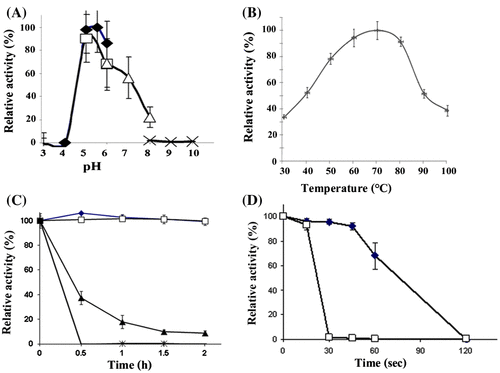
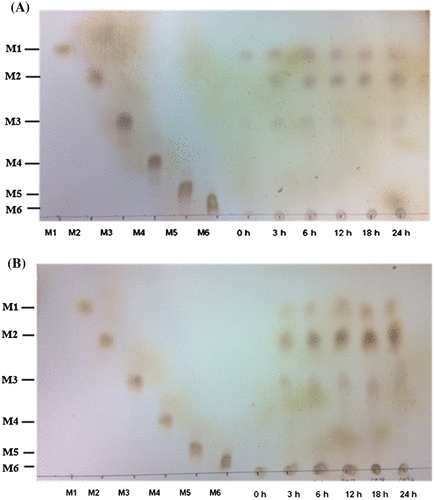
Fig. 2. Hydrolysis of PKM and CM by the recombinant BCC4525 β-mannanase for MOS production. Hydrolysis of PKM (A) and CM (B) was carried out at various time intervals before the reaction products were detected by TLC.