Figures & data
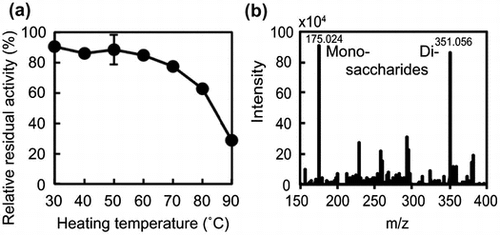
Fig. 1. Results of SDS-PAGE and the AlgC-PL7 amino acid sequence.
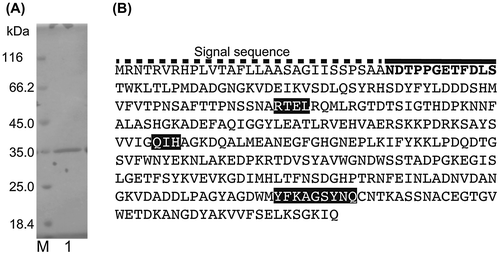
Fig. 2. Effects of temperature, pH, and salt on AlgC-PL7 activity.
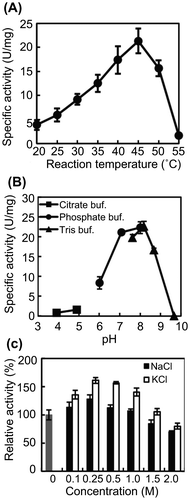
Fig. 3. Thermostability of Cobetia sp. NAP1-produced AlgC-PL7 and recombinant AlgC-PL7.
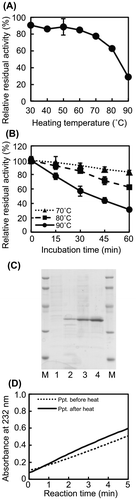
Fig. 4. Substrate specificity of Cobetia sp. NAP1-produced AlgC-PL7 (A) and recombinant AlgC-PL7 (B).
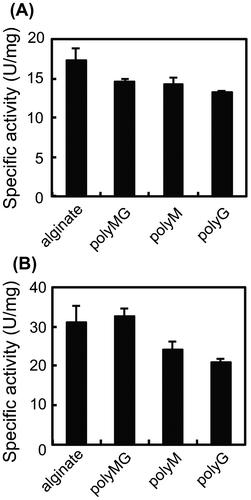
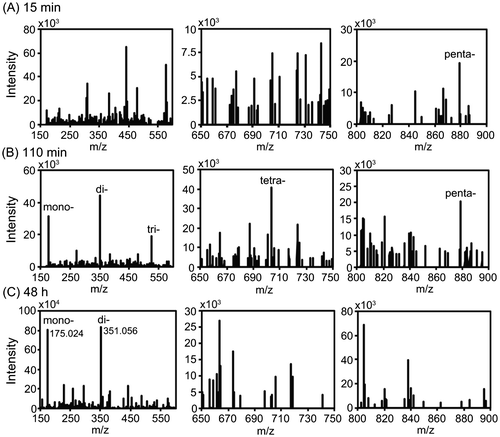
Fig. 5. ESI-MS analysis of the degradation products resulting from AlgC-PL7 activity.